ABSTRACT
Crusted (Norwegian) scabies is a rare variant of Scabies which usually presents in patients with poor cellular immunity that occurs in conditions like AIDS, Leukemia, Lymphoma, Steroid/Chemotherapy, solid organ transplant, and malnutrition. We present a case of Norwegian (crusted) scabies in a patient with concurrent leukemia cutis. The scabies infection presented with a similar rash as the leukemia cutis which delayed the diagnosis and treatment.
1. Introduction
Norwegian (crusted) scabies is a severe variant of scabies seen among immunocompromised patients. It is commonly seen with leukemia and lymphoma group of neoplasms. Given the rise of immunocompromised patients secondary to chemotherapy and immunomodulators, it is important to recognize this rare but now increasingly common dermatological condition which is often misdiagnosed and leads to increased patient suffering and mismanagement [Citation1]. We present a patient with Chronic Lymphocytic Leukemia (CLL) and leukemia cutis, who subsequently developed Norwegian scabies.
2. Case
Our patient is a 62 male with a past medical history significant for hypertension, uncontrolled type 2 DM, and eczema. He developed a generalized pruritic rash around a month prior to presentation, that started on his thighs and eventually progressed to involve his back, arms, chest, abdomen, and groin. He also had some intermittent dizziness, but no other significant symptoms and he was on no new medications.
He came to the Emergency Department (ED) when an episode of dizziness did not resolve and lasted for more than 25 minutes. He had associated left-hand numbness with the episode. He was found to be hypertensive with a blood pressure of 232/121 mm hg and with a blood sugar of 468. He was given IV hydralazine which rapidly lowered the blood pressure to 98/67 mm hg. His symptoms worsened at this point with left upper extremity weakness and tingling sensation on the left side of his face. On neurological exam, he had a left-sided weakness (4/5 strength) and numbness in both upper and lower extremities. He also had a dense left-sided hemianopsia that he was unaware of. CT head revealed an acute ischemic stroke in the distribution of the right posterior cerebral artery affecting the right occipital lobe. He was admitted to the hospital for a stroke workup.
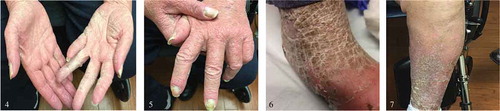
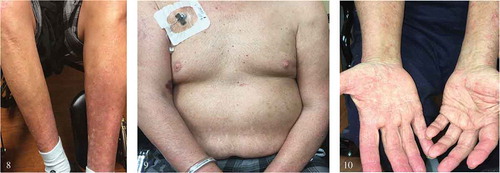
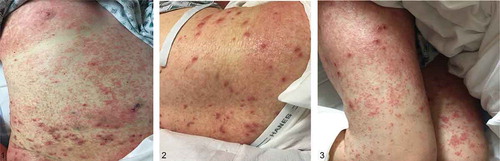
Skin exam was notable for generalized dense, symmetrical eruption composed of innumerable, erythematous papules with rough branny scale, forming scaly bumpy somewhat eczematous patches on many areas. Superimposed on this were scattered individual pustules, most notably on the thighs. No discharge was noted ().
Differential diagnosis initially included diagnoses that could explain the rash and the stroke. These included vasculitic syndromes like cryoglobulinemia, Henoch-Schonlein purpura, Churg Strauss, primary CNS vasculitis; Infectious causes like endocarditis leading to septic emboli causing strokes and malignancies causing hypercoagulable states. The stroke and the rash could not have been related, so we also considered differential diagnosis exclusively for the kind of diffuse rash he presented with like Drug eruption, DRESS syndrome, psoriasis, contact dermatitis/eczema, and infectious etiologies.
Labs were significant for persistent lymphocyte predominant leukocytosis and peripheral eosinophilia (absolute count of 1.38). Peripheral smear showed some smudge cells. Viral serologies were negative for HSV, EBV, HIV, Hep C. Vasculitic workup was negative: normal ANA, negative lupus anticoagulant, and cardiolipin antibodies. IgE and IgA levels were normal. Inflammatory markers: normal ESR (39) but CRP was elevated at 4.8 mg/L. Blood cultures were negative. TTE and TEE did not reveal any PFOs or vegetations concerning for infective endocarditis.
Steroids were started for the rash with no improvement. Antibiotics were eventually started for possible superinfection of the underlying rash. Skin biopsy was initially held off to see if the rash would resolve with steroids. Due to the persistent leukocytosis and smudge cells seen on the peripheral smear, flow cytometry was done which revealed 22% of CD5+ monoclonal B cells and no abnormal T cell or myeloid cell population. A subsequent bone marrow biopsy was consistent with chronic lymphocytic leukemia/small lymphocytic lymphoma involving an estimated 35% of the bone marrow cellular population by morphologic evaluation and 14% of the bone marrow cellular population by flow cytometric analysis. There was also peripheral eosinophilia (10%).
Skin Biopsy was done eventually which showed spongiotic dermatitis with brisk superficial and deep dermal mononuclear cell infiltrate including eosinophils and an atypical lymphocytic component, consistent with involvement by CLL/SLL. No histologic evidence of superficial infestation by mites (multiple levels through the block examined). There was no evidence of primary vasculitis in any of the specimens. The differential diagnosis of spongiotic dermatitis with a brisk dermal mixed inflammatory infiltrate containing numerous eosinophils includes hypersensitivity reactions, such as a scabies infection, arthropod bite reaction, or a drug reaction. But the presence of the atypical lymphocytic infiltrate was thought to be more consistent with CLL/SLL. This confirmed the diagnosis of Leukemia Cutis.
Given that the rash was severe and was the only manifestation of his CLL, he was treated with 3 cycles of chemotherapy (FCR – Fludarabine, Cytoxan, and Rituxan). His skin’s top layer sloughed off and vastly improved within hours of his first dose of Rituximab.
After his second cycle of chemotherapy, the rash reappeared which was initially similar to the leukemia cutis. However, it did not resolve with subsequent cycles of chemotherapy. He had several admissions for MRSA bacteremia and aortic valve endocarditis, osteomyelitis of his left foot, and hemorrhagic cystitis from cyclophosphamide during the next few months. Chemotherapy was stopped due to the side effects and his active infections.
He was started on topical steroid cream for the worsening rash by the dermatologist which did not offer much relief. The rash eventually progressed over the next 6 months into an intensely pruritic hyperkeratotic plaque with diffuse desquamation and crusting of the palms, finger webs, and shins ().
A repeat skin biopsy revealed millions of scabies mites confirming crusted (Norwegian scabies). There was no obvious infiltration of the skin by the CLL. The patient was treated with topical permethrin and oral ivermectin which led to the resolution of the skin rash over the next month ().
3. Discussion
Sarcoptes Scabiei var. hominis is an obligate ectoparasite that is known to cause two different forms of infections in terms of severity, symptoms, infectious capabilities, and the type of population affected [Citation2,Citation3]. It is a major worldwide public health issue affecting as many as 200 million people at any time as per the WHO [Citation4]. It is also notorious for masquerading as other dermatological conditions like eczema, psoriasis, seborrheic dermatitis, lichen planus, and in this case leukemia cutis [Citation1].
Non-crusted (normal/classical) scabies (usually involves 10–15 mites) is a common dermatological condition that is transmitted by the transfer of impregnated female mites through direct contact (skin-to-skin) or even fomites (bedding, towels). It mostly affects immunocompetent hosts. Although classical scabies can be easily treatable, an infected person can be asymptomatic for up to 4–6 weeks but can continue to transmit the disease actively to other people. The female mite burrows under the skin (stratum corneum) and is found in the finger webs, wrist, elbow, and knee folds. The resultant maculopapular rash with scaling and intense pruritis is often an immune/allergic response to the presence of mites, eggs, and feces on the skin [Citation3,Citation5].
Crusted (Norwegian) Scabies, however, is a rare variant that was first described in Norway in leprosy patients (hence the name). It is typically seen in conditions with a defective T-cell immune response like HIV and Adult T cell leukemia in HTLV-1 seropositive patients [Citation2]. It can also present in patients with hematological malignancies like lymphomas and in immunosuppressive states that occur in nutritional deficiencies, chronic alcoholism, and long-term steroid use [Citation1]. Patients with the crusted scabies have thousands to millions of mites on the skin making it highly contagious. The mites multiply in the millions as a result of the immunosuppression rather than increased virulence [Citation2]. It can be transmitted through fomites, exfoliated skin, or brief contact with an infected individual. It usually manifests as an extensive thick, hyperkeratotic, crusted, plaque-like scaly lesions distributed on the extremities, back, face, scalp, web spaces, and nail folds. It can often involve the fingernails and toenails with a thickened yellowish appearance. In contrast to classical scabies, Norwegian scabies can be non-pruritic which can delay the diagnosis [Citation1,Citation2]. Labs often reveal peripheral eosinophilia and increased Ig E levels. It is associated with a high mortality rate due to secondary bacterial superinfection like impetigo leading to post-streptococcal glomerulonephritis and sepsis [Citation6].
Our patient was immunocompromised due to his underlying CLL and chemotherapy. The fact that the initial rash resolved after the first dose of chemotherapy makes it clear that our patient initially had leukemia cutis, which is the leukemic involvement of the skin. The 2nd time a similar rash appeared it was found to be due to crusted scabies. No leukemic infiltration of the skin was found at that time.
Figures 1, 2, and 3. demonstrate the leukemia cutis rash – generalized dense, symmetrical eruption composed of innumerable, erythematous papules with rough branny scale, forming scaly bumpy somewhat eczematous patches on many areas. Superimposed on these were scattered individual pustules, most notably on the thighs
There are few case reports that report concurrent leukemic involvement of the skin and crusted scabies [Citation7,Citation8]. Treatment of scabies resulted in the resolution of the skin lesions [Citation7]. In our literature search, we could not find a similar case like ours that report initial leukemia cutis with resolution from chemotherapy and recurrence of similar rash due to crusted scabies. It is possible that the scabies mites were missed in the initial presentation although we think it is unlikely as multiple blocks of skin were biopsied and did not reveal scabies mites. The immunocompromise and the skin affected by the leukemic infiltrate probably acted as a breeding ground for the scabies mites which later presented as crusted scabies.
Often the diagnosis is delayed as crusted scabies masquerade as other diseases as was the case in the patient we presented. We suspected a relapse of the leukemia cutis when the patient had a recurrent rash which delayed the diagnosis and treatment for scabies. If not identified and appropriately treated on time, the lesions can eventually become exophytic and verrucous making it difficult to treat with just topical therapy due to poor penetration [Citation2]. Diagnosis is made by clinical symptoms and microscopy of skin biopsy/scrapings revealing the scabies mites. Most often, inflammatory cell infiltrates comprising eosinophils, lymphocytes, and histiocytes are also found given the immune reaction to the mites. The duration of treatment is often longer in crusted scabies requiring both oral ivermectin (not FDA approved) and 5% permethrin cream for up to 1–2 months, as was the case with our patient [Citation2].
4. Conclusion
A chronic, recurrent hyperkeratotic, crusted lesion unresponsive to treatment in an immunocompromised individual, especially with defective T-cell immunity should raise suspicion for Norwegian scabies. It can occur concurrently in a patient with leukemia cutis or present with a similar rash after the resolution of the leukemic infiltrates. Diagnosis can be delayed as it can masquerade as other dermatological conditions.
Aknowlegement
We would like to thank Ms. Darcie Cote-Rumsey, medical librarian at Greater Baltimore Medical Center, for her help with the extensive literature search.
Disclosure statement
No potential conflict of interest was reported by the authors.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
References
- Grabowski G, Kanhai A, Grabowski R, et al. Norwegian scabies in the immunocompromised patient. J Am Podiatr Med Assoc. 2004 November/December;94(6):583–586. .
- Ghosh T, Jandhyala D, Bhatti MM, et al. The brief case: crusted scabies in a leukemic patient following a stay in a long-term acute care facility. J Clin Microbiol. 2017 May;55(5):3006–3015.
- Walton SF and Currie BJ. Problems in diagnosing scabies, a global disease in human and animal populations American society for microbiology. Clin Microbiol Rev. 2007 Apr;20:268–279.
- World Health Organization. 2016. Neglected tropical diseases. Geneva, Switzerland: World Health Organization. Available from: http://www.who.int/neglected _diseases/diseases/en/.
- Centers for Disease Control and Prevention. DPDx. Scabies. Division of parasitic diseases and malaria. Atlanta, GA: CDC; 2013 November 29. Available from: http://www.cdc.gov/dpdx/scabies/.
- Tanaka T, and Usuba M, etal.. Scabies norvegica associated with leukemia cutis (chronic myeloid leukemia) Tohuku. J Exper Med. 1960;72:35–41.
- Dostrovsky A, Raubitschek F, Sagher F, et al. Scabies Norvegia and lymphatic leukemia with report of a hospital epidemic of atypical scabies. Dermatologica. 1956;113:26.
- Kandi V. Laboratory diagnosis of scabies using a simple saline mount: a clinical microbiologist’s report. Cureus. 2017March 19;9(3):e1102.Centers for Disease Control and Prevention. DPDx. Scabies. Division of parasitic diseases and malaria. Atlanta, GA: CDC; 2013 November 29. Available from: .