ABSTRACT
Pyoderma gangrenosum (PG) is an inflammatory skin condition that is often misdiagnosed as a necrotizing infection. This diagnosis must be considered in any patient with underlying systemic disease who presents with large ulcerating lesions that are unresponsive to antibiotics. Early diagnosis and a multidisciplinary approach to treatment are crucial to achieving improvement in quality of life and minimizing cosmetic morbidity.
1. Introduction
Pyoderma gangrenosum (PG) is a rare inflammatory skin condition with an annual incidence of 3–10 cases in one million people. The pathogenesis is unclear but involves aberrant neutrophil activity [Citation1] as well as a genetic component [Citation2]. Underlying systemic disease is present in 50–70% of cases [Citation3], and includes hematological disorders, inflammatory bowel disease (IBD) and rheumatoid arthritis. It can manifest initially as a papule, pustule, or vesicle that progresses to a painful ulcer [Citation4]. PG is often misdiagnosed as a skin and soft tissue infection and inappropriately treated with antibiotics with no benefit. This may prompt intervention with surgical debridement, which frequently leads to a vicious cycle of worsening necrosis given the strong association of PG with severe pathergy phenomenon. Significant morbidity can be prevented by early diagnosis and treatment [Citation5]. PG of the breast is rare, with only 43 reported cases, 70% of which developed after breast surgery [Citation6]. The most frequently involved areas are the lower extremities including the pretibial area. The trunk, head and neck, and peristomal skin are less frequently affected.
2. Case description
We present the case of a 46 year old woman with a history of Crohn’s disease on mesalamine and iridocyclitis, who presented with left breast pain. She first noticed mild skin irritation which she attributed to the presence of her undergarment, but a small pimple subsequently developed and continued to progress to a reddish/bluish discoloration with brown discharge, then became swollen. Four days after the initial onset, she presented to the emergency department with severe breast pain. She denied constitutional symptoms such as fevers, chills and weight loss. She reported a normal mammogram 6 months prior. On presentation, she was tachycardic at 118 bpm and hypertensive at 147/98. She was afebrile with a normal respiratory rate. Breast examination revealed edema and erythema with skin breakdown and significant tenderness on palpation.
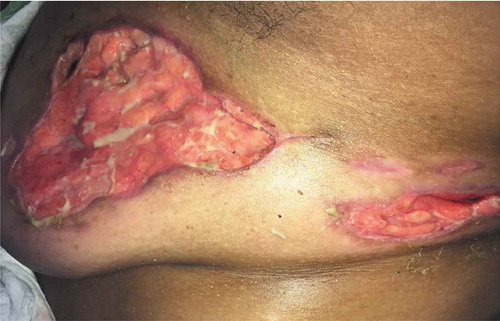
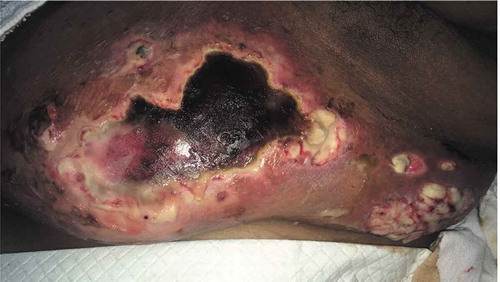
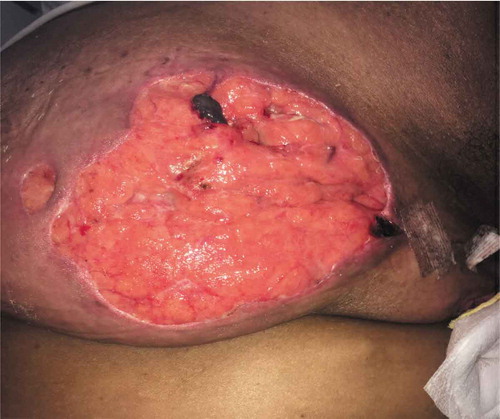
Laboratory investigations revealed a leukocytosis of 15.02 x 103/µL (4–11x103/µL). A contrast enhanced CT of the chest showed extensive stranding and soft tissue density throughout the left breast, compatible with extensive cellulitis. Wound cultures were obtained and antibiotic therapy was initiated with vancomycin and metronidazole. This was later narrowed to clindamycin, then switched to ceftaroline after wound cultures returned positive for methicillin resistant staphylococcus aureus and Cronobacter sakazakii complex. Within 2 days the lesion progressed to a large punched out full thickness ulcer with serpiginous and erythematous edges, with exposure of the lobular breast tissue (Picture 1–3).
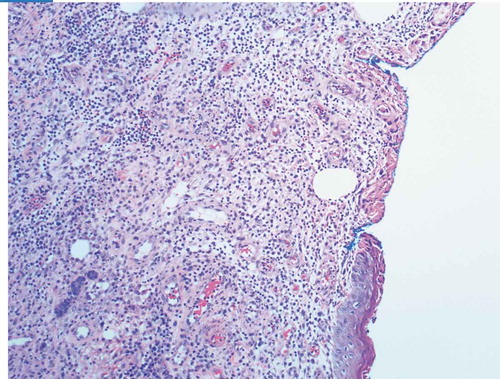
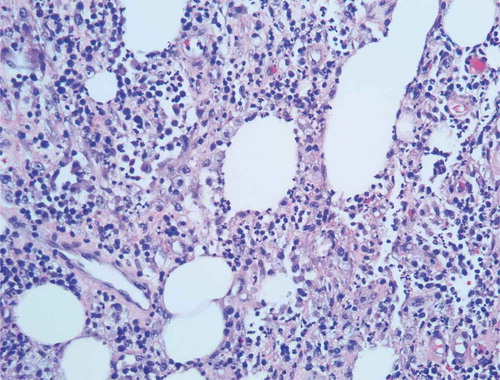
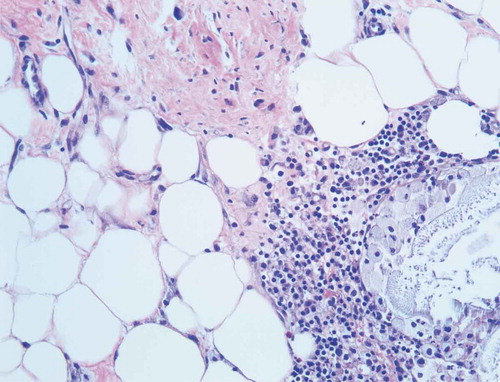
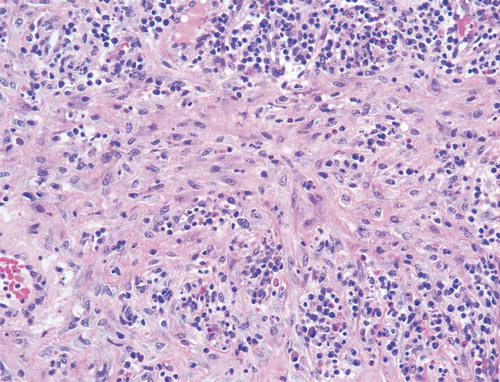
Given the progression of the lesions while on appropriate culture-directed antibiotics in the setting of a history of Crohn’s disease, the differential was broadened to include PG and oral prednisone was initiated. A skin biopsy showed acute and chronic inflammation extending into the subcutaneous adipose tissue, with collections of neutrophils with necrosis and histiocytic activity. These findings were non-specific but were consistent with PG given the clinical picture (Picture 5–8).
The underlying predisposing factor in this patient was thought to be Crohn’s disease, but further workup was done to exclude other causes. Antinuclear antibody, rheumatoid factor, and a hepatitis panel were negative. As PG was most likely an extraintestinal manifestation of her underlying Crohn’s disease, she underwent esophagogastroduodenoscopy and colonoscopy for re-staging. Findings were negative for evidence of worsening GI inflammation.
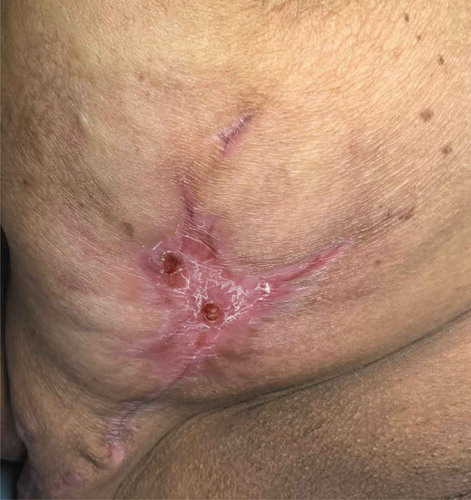
She was treated with prednisone 60 mg for 1 week with a slow taper, decreasing by 10 mg every week. On completion of the steroid taper, Infliximab 10 mg/kg q 8 week was started outpatient as a steroid-sparing agent, which led to complete wound healing with some scarring. Picture 4
3. Discussion
PG is the second most common dermatologic manifestation of IBD after erythema nodosum. A prospective cohort study by Farhi et al. in 2008 found a prevalence of 0.75% among 2402 patients with IBD [Citation7]. The relationship between the underlying disease activity of IBD and the development of PG is controversial, but there are data to support the school of thought that the development of PG is independent of disease activity [Citation8], as was demonstrated in this case. This is an important point to remember, as the presence of underlying systemic disease can be overlooked if it is inactive, delaying recognition of PG.
PG is a diagnosis of exclusion and the skin manifestations can be mistaken for a variety of infectious and non-infectious etiologies. These include cutaneous tuberculosis, leishmaniasis, sporotrichosis or other fungal infections, vasculitis, malignancy, or thrombophilias [Citation9–11]. The diagnosis is often clinical, but skin biopsy, while not diagnostic, can help support the diagnosis, and typically reveals a dermal neutrophilic infiltrate. In practice, the initial workup of the typical presenting lesions includes wound cultures which usually grow skin commensals, most commonly staphylococcus. This prompts antibiotic therapy, which may be associated with worsening of the skin condition as the underlying disease process remains untreated. A delay in making the correct diagnosis can lead to inappropriate debridement which leads to progressive enlargement of the ulcers with deep tissue involvement due to pathergy phenomenon. With prolonged unrecognized disease, wound cultures may reveal growth of more resistant organisms such as pseudomonas aeruginosa [Citation5].
Recently published criteria by Maverakis et al., 2018 can aid in making the diagnosis of PG (). [Citation9]. Consideration of PG is not only important in the presence of known underlying systemic disease but can also be the initial presenting manifestation and so should always be on the differential in any patient [Citation12]..
Table 1. Two cases of copper deficiency due to malabsorption leading to myeloneuropathy
Table 2. Diagnostic criteria for pyoderma gangrenosum [Citation9]
A lack of established guidelines makes treatment of PG challenging as there are few clinical trials in the literature. The initial approach for treatment should involve reducing inflammation, optimizing wound healing, and identifying and treating the underlying disease. For silent disease evidence-based symptomatic treatment must come into play [Citation13].
The severity of disease guides treatment. For mild disease, topical agents like high potency corticosteroids or calcineurin inhibitors are usually adequate. In a prospective cohort study of 66 patients by Thomas et al., ulcer healing occurred in 43.8% at 6 months with clobetasol propionate 0.05%, with recurrence in 15%. For severe disease, systemic treatment with either corticosteroids or cyclosporine is recommended as first-line therapy. A randomized controlled trial by Ormerod et al., 2015 on 121 patients found oral prednisolone and cyclosporine to be equivalent, with ulcer healing noted in 47% of participants in both the cyclosporine and prednisolone groups at 6 months. Recurrence occurred in 30% of those receiving cyclosporine and 28% of those on prednisolone [Citation14]. Second-line therapies include anti-TNF medications (including infliximab as used in this case), intravenous immunoglobulins, methotrexate, azathioprine, cyclophosphamide, dapsone, thalidomide, or mycophenolate mofetil. Increasing evidence supports the use of anti-TNF medications such as infliximab, etanercept, and adalimumab. Newer biological agents ustekinumab (anti-IL-23), ixekizumab (anti-IL-17), and brodalumab (anti-IL-17 R) have shown promising results as well [Citation15].
Learning Points:
Pyoderma gangrenosum (PG) is a rare ulcerative and inflammatory skin condition that is frequently misdiagnosed, leading to a delay in appropriate treatment and significantly increased cosmetic morbidity.
A high index of suspicion and the use of recently published diagnostic criteria can help clinicians to diagnose and treat PG early in the disease course.
Ulcerative lesions not responding to antibiotics and worsening of lesions after surgical debridement are some important clues to the diagnosis.
We recommend early skin biopsy and appropriate treatment with local or systemic steroids, calcineurin inhibitors, cyclosporine or other immunosuppressants.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Adachi Y, Kindzelskii AL, Cookingham G, et al., Todd RF 3rd. Aberrant neutrophil trafficking and metabolic oscillations in severe pyoderma gangrenosum. Petty HRJ Invest Dermatol. 1998;111(2):259.
- Shands JW Jr, Flowers FP, Hill HM, et al. Pyoderma gangrenosum in a kindred. Precipitation by surgery or mild physical trauma. J Am Acad Dermatol. 1987;16(5 Pt 1):931–934.
- Wollina U. Pyoderma gangrenosum–a review. Orphanet J Rare Dis. 2007;2:19.
- Callen JP, Jackson JM. Pyoderma gangrenosum: an update. Rheum Dis Clin North Am. 2007;33(4):787–802.
- Saffie MG, Shroff A. A case of pyoderma gangrenosum misdiagnosed as necrotizing infection: a potential diagnostic catastrophe. Case Rep Infect Dis. 2018;(2018:8907542.
- Marinopoulos S, Theofanakis C, Zacharouli T, et al. Pyoderma Gangrenosum of the breast: A case report study. Int J Surg Case Rep. 2017;31:203–205.
- Farhi D, Cosnes J, Zizi N, et al. Significance of erythema nodosum and pyoderma gangrenosum in inflammatory bowel diseases: a cohort study of 2402 patients. Medicine (Baltimore). 2008;87(5):281–293.
- Thornton JR, Teague RH, Low-Beer TS, et al. Pyoderma gangrenosum and ulcerative colitis. Gut. 1980;21(3):247–248.
- Maverakis EM, Ma C, Shinkai K, et al. Diagnostic criteria of ulcerative pyoderma gangrenosum a delphi consensus of international experts. JAMA Dermatol. 2018;154(4):461–466.
- Su WP, Davis MD, Weenig RH, et al. Pyoderma gangrenosum, clinicopathologic correlation and proposed diagnostic criteria. Int J Dermatol. 2004;43(11):790–800.
- Weenig RH, Davis MD, Dahl PR, et al. Skin ulcers misdiagnosed as pyoderma gangrenosum. N Engl J Med. 2002;347(18):1412–1418.
- Shahid S, Myszor M, De Silva A. Pyoderma gangrenosum as a first presentation of inflammatory bowel disease. BMJ Case Rep. 2014;(2014:bcr2014204853.
- Ahronowitz I, Harp J, Shinkai K. Etiology and management of pyoderma gangrenosum: a comprehensive review. Am J Clin Dermatol. 2012;13(3):191–211.
- Ormerod AD, Thomas KS, Craig FE, et al., UK Dermatology Clinical, Trials Network’s STOP GAP Team. Comparison of the two most commonly used treatments for pyoderma gangrenosum: results of the STOP GAP randomised controlled trial. BMJ. 2015;350: h2958.
- Patel F, Fitzmaurice S, Duong C, et al. Effective strategies for the management of pyoderma gangrenosum: a comprehensive review. Acta Derm Venereol. 2015;95(5):525–531.