ABSTRACT
Sarcoidosis is a systemic inflammatory condition causing increased immune system activity and manifesting as noncaseating granulomatous disease with the ability to affect multiple organ systems. Neurosarcoidosis is an uncommon presentation, with just 5–10% of patients with sarcoidosis experiencing intracranial disease. The diagnosis of neurosarcoidosis can be difficult, especially given the overlap of imaging findings with more common intracranial lesions. This case presents trigeminal neuralgia as the initial symptom of neurosarcoidosis and emphasizes the importance of a high clinical index of suspicion for neurosarcoidosis in patients with otherwise unexplained neurological symptoms.
1. Introduction
Sarcoidosis is a heterogenous systemic immune activation disease which involves multiple organ systems [Citation1–3]. The pathophysiology of sarcoidosis is related to the formation of noncaseating granulomas characterized by macrophages, epithelioid cells, multinucleated giant cells, and CD4 + T-lymphocytes [Citation1–4]. Proposed etiologies are multifactorial, involving both genetic predisposition and environmental exposures [Citation1,Citation2]. Nearly 90% of cases exhibit pulmonary involvement, which accounts for the majority of case morbidity and mortality; bilateral hilar adenopathy is a classical imaging finding [Citation1–3]. Common presenting symptoms include cough, skin manifestations, uveitis, peripheral lymphadenopathy, and fatigue; incidental finding of hilar adenopathy on chest radiograph has also been reported [Citation1].
Neurologic complications occur in approximately 5–10% of patients diagnosed with sarcoidosis, and most commonly manifest with cranial nerve involvement, headache, and seizure [Citation5,Citation6]. Cranial nerve involvement occurs most often in the facial nerve (related to parotid gland inflammation) or the optic nerve [Citation5,Citation7,Citation8]. Less commonly the trigeminal, auditory, or cochlear nerves may be involved [Citation5,Citation7,Citation9–14]. Optimal imaging for evaluation of neurosarcoidosis is magnetic resonance imaging (MRI) of the brain with gadolinium contrast, which demonstrates granulomatous infiltration of neural tissue [Citation6,Citation15,Citation16]. Common MRI findings of neurosarcoidosis include predilection for the leptomeninges, usually seeing homogenous nodular or diffuse enhanced thickening of the affected meninges [Citation5,Citation7,Citation16]. There are no specific imaging features to differentiate trigeminal sarcoidosis from other more common intracranial findings related to the trigeminal nerve, such as schwannoma and meningioma, and imaging features may resemble multiple sclerosis over time with lesions that occur and regress at varying intracranial locations [Citation5]. If intracranial tissue biopsy is not feasible, extracranial tissue biopsy should be pursued when sarcoidosis is a possible diagnosis [Citation1,Citation2].
This case involves trigeminal nerve neurosarcoidosis presenting as trigeminal neuralgia with imaging findings suggestive of trigeminal schwannoma, necessitating extracranial tissue biopsy for diagnosis.
2. Case report
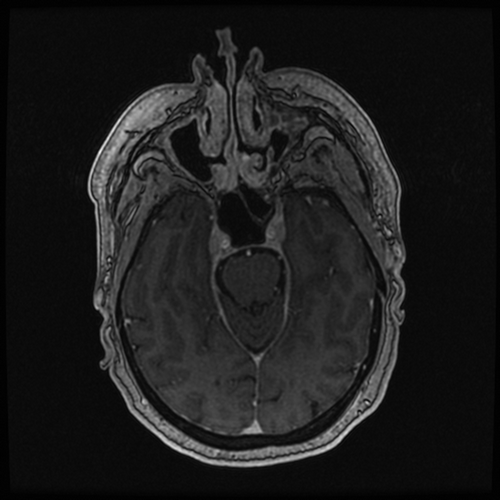
A 53 year old woman with a past medical history of asthma, anxiety, depression, and mitral valve prolapse presented to her primary care office for 3 weeks of right-sided facial numbness. Her symptoms started abruptly and were associated with jaw weakness, difficulty eating, and one episode of transient diplopia. Neurological exam was remarkable for a sluggish response to direct light reflex in the left pupil, reduced sensation to light touch and loss of 2-point discrimination in the right V2 distribution of the fifth cranial nerve, and asymmetric smile with diminished left nasolabial fold. There were no other nerve palsies. Deep tendon reflexes were symmetrically brisk throughout without clonus. Magnetic resonance imaging (MRI) of the brain with and without contrast and blood work was ordered. Laboratory studies revealed normal inflammatory markers, negative Lyme antibody serologies, normal thyroid-stimulating hormone (TSH) level, and unremarkable complete metabolic panel (CMP). Brain MRI revealed a homogeneously enhancing 18x11x8mm mass centered in the right Meckel’s cave with enhancement coursing along the V2 and V3 segments of the right fifth cranial nerve, most consistent with a trigeminal schwannoma (). Meningioma was also considered, with less likely etiologies being metastatic disease, neurosarcoidosis, or lymphoma.
Figure 1. MRI brain T1 sequence with contrast. Centered in the right Meckel’s cave is a homogeneously enhancing 18 × 11 x 8 mm mass with enhancement coursing along the V2 and V3 segments of the right 5th cranial nerve
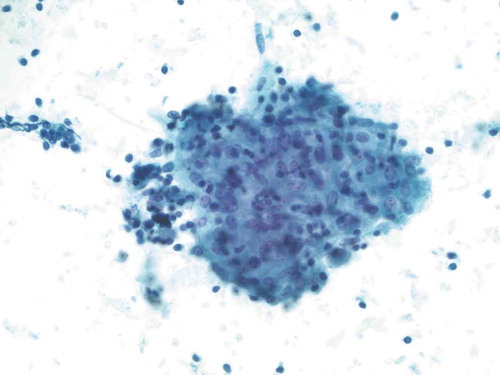
The patient was referred to neurosurgery at a tertiary center and was seen 2 months after her initial presentation. Symptoms were completely resolved at that time. Further workup revealed serum ACE level in the high range of normal at 64 (mcg/L) (normal range 9–67 mcg/L) and negative tuberculosis testing. Chest radiograph showed mild soft tissue prominence of the right hilum suspicious for artifact versus adenopathy. Subsequent CT chest was significant for nonspecific mediastinal and bilateral hilar adenopathy, with malignancy being the main consideration and sarcoidosis possible based on imaging characteristics. The patient remained asymptomatic and was referred to pulmonology, approximately 3 months after her initial presentation. Pathological examination of endobronchial ultrasound (EBUS) transbronchial biopsy showed noncaseating granulomas consistent with sarcoidosis (); the biopsy was negative for malignancy. Bronchoalveolar lavage cultures were negative for bacterial or fungal growth. A diagnosis of sarcoidosis was made; however, no treatment was pursued at that time given the lack of clinical manifestations and symptoms.
Figure 2. Smear preparation showing cohesive clusters of epithelioid cells, scattered background small lymphocytes (Papanicolaou stain, 200x original magnification)
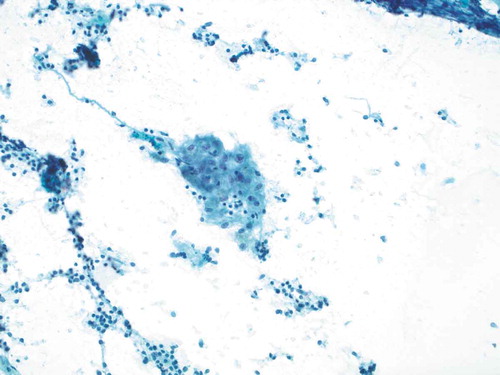
Figure 3. Smear preparation showing cohesive clusters of epithelioid cells, scattered background small lymphocytes (Papanicolaou stain, 200x original magnification)
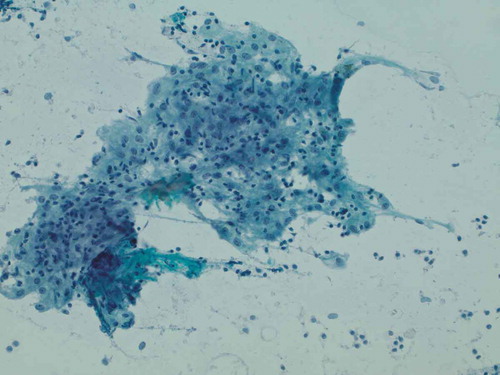
Figure 4. Representative epithelioid cellular cluster showing architectural cohesion, indistinct cytoplasmic borders, and uniform-appearing nuclei with minute nucleoli, consistent with non-caseating granuloma (Papanicolaou stain, 400x original magnification)
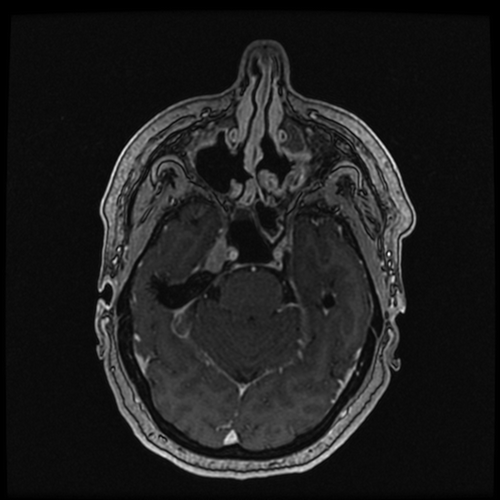
Follow up brain MRI was obtained 6 months after initial presentation, showing an interval decrease in the pathologic enhancement involving the right Meckel’s cave, with new enhancement in the left Meckel’s cave measuring 12 × 7 mm (). Given the biopsy-confirmed diagnosis of sarcoidosis, the brain lesions were determined to be secondary to neurosarcoidosis. The patient developed new symptoms of headache and nausea and was started on 1.5 mg/kg/day of prednisone (100 mg daily) for treatment. Approximately 3 days after initiation of steroids, the patient’s symptoms were resolved. No neurosurgical intervention was deemed necessary, and consideration for immunosuppressive therapy in the future was proposed.
3. Discussion
Neurosarcoidosis causes inflammatory effects within the central nervous system and has been found to be present in approximately 5–10% of patients diagnosed with sarcoidosis [Citation5,Citation6]. The patient in this case presented with trigeminal neuralgia, suspicious for Lyme disease versus multiple sclerosis, and warranting brain MRI for further evaluation. Although MRI is the preferred imaging modality for neurosarcoidosis evaluation, the diagnosis was complicated as imaging findings in neurosarcoidosis overlap with more common diagnoses such as schwannoma, meningioma, neurofibroma, and other inflammatory lesions [Citation5,Citation7,Citation16]. Evaluating for other manifestations of sarcoidosis was necessary given the possibility of sarcoidosis and diagnostic uncertainty. Significant findings on chest radiograph and chest CT prompted the performance of biopsy which ultimately led to the appropriate diagnosis of neurosarcoidosis. Proposed diagnostic criteria for neurosarcoidosis include suggestive clinical and radiographic presentation, biopsy of either intracranial or extracranial tissue showing non-caseating granulomas, and no alternative diagnosis [Citation1,Citation2], all of which are demonstrated in this case. Of note, serum ACE levels are often obtained in the workup for sarcoidosis but do not have good sensitivity or specificity for diagnosis [Citation1,Citation5].
Treatment for neurosarcoidosis is generally based on high dose steroid regimens [Citation5,Citation6,Citation15]. Many cases are refractory or recur despite steroid use, necessitating the use of other medications, usually immunomodulatory and cytotoxic therapies such as methotrexate, hydroxychloroquine, azathioprine, or cyclosporine. These treatments have been shown to be of potential benefit for patients who do not improve with or relapse after steroid treatment [Citation5,Citation15]. This patient responded early and well to weight-based oral prednisone with quick resolution of her symptoms.
Neurosarcoidosis remains a difficult diagnosis to make based solely on presenting symptoms and initial imaging and laboratory findings. This case emphasizes the importance of obtaining a complete workup for sarcoidosis if the diagnosis is in question, including chest imaging and tissue biopsies for pathological evaluation. As described in prior case reports, neurosarcoidosis may be isolated to the CNS without extracranial manifestations [Citation9–14], and full workup with obtaining intracranial tissue samples should still be pursued in these cases. The internist should maintain a high clinical index of suspicion for the possibility of sarcoidosis as the diagnosis in patients presenting with unexplained neurological symptoms, even in the absence of typical pulmonary manifestations.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Valeyre D, Prasse A, Nunes H, et al. Sarcoidosis. Lancet. 2014;383(9923):1155–1167.
- Ma Y, Gal A, Koss MN. The pathology of pulmonary sarcoidosis: update. Semin Diagn Pathol. 2007;24(3):150–161.
- Rossi G, Cavazza A, Colby TV. Pathology of sarcoidosis. Clin Rev Allergy Immunol. 2015;49(1):36–44.
- Baughman RP, Lower EE, Du Bois RM. Sarcoidosis. Lancet. 2003;361(9363):1111–1118.
- Nowak DA, Widenka DC. Neurosarcoidosis: a review of its intracranial manifestation. J Neurol. 2001;248(5):363–372.
- Burns TM. Neurosarcoidosis. Arch Neurol. 2003;60(8):1166–1168.
- Carlson ML, White JR Jr, Espahbodi M, et al. Cranial base manifestations of neurosarcoidosis: a review of 305 patients. Otol Neruotol. 2015;36(1):156–166.
- Stern BJ, Krumholz A, Johns C, et al. Sarcoidosis and its neurological manifestations. Arch Neurol. 1985;42(9):909–917.
- Amin A, Balderacchi JL. Trigeminal neurosarcoidosis: case report and literature review. Ear Nose Throat J. 2010;89(7):320–322.
- Arias M, Iglesias A, Vila O, et al. MR imaging findings of neurosarcoidosis of the gasserian ganglion: an unusual presentation. Eur Radiol. 2002;12(11):2723–2725.
- Bangiyev L, Kornacki S, Mikolaenko I. Rare isolated trigeminal nerve sarcoidosis mimicking schwannoma. Clin Imaging. 2015;39(1):133–135.
- Bonnet F, Mercie P, Morlat P, et al. Isolated involvement of the trigeminal nerve of sarcoidosis origin. Rev Neurol. 1997;153(1):59–61.
- Braksick S, Shah-Haque S, El-Haddad B, et al. Neurosarcoidosis presenting as trigeminal nevralgia: a case report and review of the literature. Sarcoidosis Vasc Diffuse Lung Dis. 2013;30(2):153–156.
- Quinones-Hinojosa A, Chang EF, Khan SA, et al. Isolated trigeminal nerve sarcoid granuloma mimicking trigeminal schwannoma: a case report. Neurosurgery. 2003;52(3):700–705.
- Joseph FG, Scolding NJ. Sarcoidosis of the nervous system. Pract Neurol. 2007;7(4):234–244.
- Terushkin V, Stern BJ, Judson MA, et al. Neurosarcoidosis: presentations and management. Neurologist. 2010;16(1):2–15.