ABSTRACT
Eptifibatide is a glycoprotein (GP) IIb/IIIa receptor antagonist, used for the treatment of acute coronary syndrome with high-risk features or ongoing ischemia. Several case reports have described thrombocytopenia as a rare side effect of eptifibatide administration. The exact mechanism remains unclear but may be due to immune destruction of circulating platelets in the peripheral blood. We present the case of acute-onset severe thrombocytopenia in a 76-year-old female undergoing percutaneous coronary intervention.
1. Introduction
Eptifibatide is a glycoprotein (GP) IIb/IIIa receptor antagonist. It is indicated for the treatment of acute coronary syndrome with high-risk features or ongoing ischemia. Eptifibatide is one of the three approved GP IIb/IIIa inhibitors in the USA, the others being abciximab and tirofiban. GP IIb/IIIa is a membrane receptor specific for platelets. When active, it binds fibrinogen to fibrinogen platelet receptor, promoting the final step of platelet aggregation causing platelet thrombi [Citation1].
Bleeding is the major side effect of GP IIb-IIIa inhibitors. A literature review identified thrombocytopenia as a rare side effect. We present a case of acute-onset severe thrombocytopenia in a 76-year-old female undergoing percutaneous coronary intervention.
2. Case presentation
A 76-year-old white female was brought in by ambulance to the Emergency Department (ED) with acute shortness of breath after suffering from intermittent retrosternal chest pressure at rest for 2 days. Her medical history was notable for coronary artery disease without cardiac stents that was treated medically with clopidogrel, diabetes mellitus, and hypertension. Her home medications included sitagliptin 100 mg once a day (QD), metformin 1000 mg two times a day, atorvastatin 20 mg QD, amlodipine 5 mg QD, losartan 50 mg QD, clopidogrel 75 mg QD, and acetaminophen 650 mg three times a day as needed. She was a non-smoker with no history of alcohol or intravenous drug abuse. The patient had a history of peptic ulcer disease while taking aspirin, and thus was taking clopidogrel as an alternative anti-platelet. While being transported via ambulance, she was found to be hypoxic at 72% and was started on noninvasive ventilation. She was also given four doses of sublingual nitroglycerin.
Upon evaluation in the ED, she had a blood pressure of 151/81 mmHg, a heart rate of 109 beats per minute, and a respiratory rate of 25 breath per minute. Her oxygen saturation was 93% with the noninvasive ventilation. Her physical exam revealed bilateral crackles on lung auscultation, and the heart exam showed a regular rate and rhythm. An initial electrocardiogram revealed sinus tachycardia with T wave inversions in the inferior and lateral leads. Her troponin was elevated at 0.5 ng/mL (normal reference range <0.01 ng/dL). Her creatine kinase myocardial band (CKMB) was elevated at 58.9 ng/mL (normal 0.6–6.3 ng/dL) and serum pro-brain Natriuretic peptide at 4443 pg/mL (normal 0–300 pg/mL).
Day 0: The patient was diagnosed with non-ST elevation myocardial infarction (NSTEMI) and was started on a heparin drip, beta-blocker, and aspirin; clopidogrel and statin were continued, and diuresis was initiated. She was admitted to the coronary care unit (CCU).
Day 1: She underwent a cardiac catheterization, which revealed severe 3-vessel coronary artery disease: Left Anterior Descending (LAD) Right Circumflex Artery (RCA), and circumflex. No intervention was performed, and the cardiothoracic surgery team was made aware of the results and the need for complete revascularization.
However, further workup, including computed tomography scan showed a severely calcified aorta, and the patient began complaining of recurrent chest pain. In the setting of ongoing ischemia, the surgery was considered high-risk.
Day 6, 19.00: A second cardiac catheterization was done. A balloon angioplasty was performed with bare metal stent placement in the lesion in the proximal circumflex. Another balloon angioplasty was performed to the lesion in the second obtuse marginal artery.
Day 6, 20.00: During the procedure, eptifibatide was initiated and continued as a drip. The patient had no prior known exposure to eptifibatide. Heparin was discontinued. The patient became hemodynamically unstable, and norepinephrine was started for blood pressure support.
Day 7, 6.40: The platelet count dropped to 3 from 243 K/uL (normal 130–400 K/uL). Eptifibatide was stopped, and aspirin and clopidogrel were continued.
Day 7, 8.00: The platelet count was repeated and confirmed to be 3 K/uL. The peripheral blood smear showed no dysplastic cells, schistocytes or spherocytes, and showed 0–2 platelets per high powered field.
The patient received one unit of platelets.
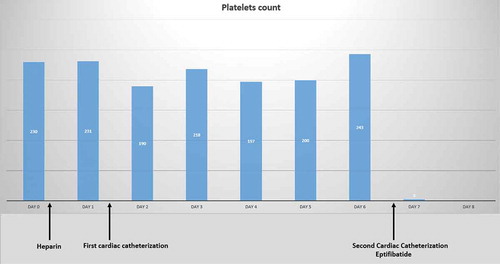
Day 7, 15.30: The platelet count increased to 78 K/uL. The platelet count trend is shown in . Further blood tests are shown in .
Table 1. Laboratory data on hospital day 7
Despite revascularization, the patient went into cardiogenic shock and dobutamine was initiated. Her condition continued to deteriorate, and her family opted for limited medical care.
Day 8, 00.34: The patient was pronounced dead.
Legend: INR, international normalized ratio; PT, prothrombin time; PTT, partial thromboplastin time; AST, aspartate amino transferase; ALT, amino alanine transferase; ADAMTS13, a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13.
3. Discussion
Thrombocytopenia is defined as a platelet count below 150,000/microL for adults. It is divided into mild (platelet count 100,000 to 150,000/microL), moderate (50,000 to 99,000/microL), and severe (<50,000/microL) [Citation2]. It is a common laboratory finding in the hospital, especially in critical care units, with an incidence ranging from 15% to 60%[Citation3].
After a comprehensive history and thorough physical exam, the first step in the workup of thrombocytopenia is to confirm the platelet count by repeating the complete blood count and checking the blood smear for clumping or giant platelets. These are two vital steps in ruling out benign pseudo-thrombocytopenia or hemodilution. It is also essential to look for other lineage abnormalities. Pseudo-thrombocytopenia is a laboratory artifact occurring when platelet aggregates go unrecognized by the automated cell counter. The clumping is caused by expression of class M immunoglobulin against epitopes on the platelets surface enhanced via the calcium chelation by ethylenediaminetetraacetic acid (EDTA). Repeating the platelet count in a citrated tube establishes the diagnosis in most cases. Rarely, a peripheral smear is required.
The next step is to review the patient’s medication list. In our patient, heparin and clopidogrel are both known to cause thrombocytopenia. Heparin exposure can generate antibodies to the complex formed by platelets factor 4 (PF4) and heparin. It consequently drops the platelet count, either acutely within the first 2 days of heparin administration, which would be a mild and usually temporary drop (also known as type I heparin-induced thrombocytopenia (HIT)) or within 5 days of exposure, which could be more serious with a risk of thrombosis (also known as type II HIT). The diagnosis of HIT is both clinical and laboratory. A high suspicion with elevated 4 T score (thrombocytopenia, temporal features, thrombosis, and checking other causes) should be supported by positive enzyme-linked immunosorbent assay that detects antibodies to PF4 [Citation4]. Our patient was on heparin drip for 7 days, so HIT was included in the differential. The patient’s 4 T score was calculated to be 3 (2 for timing, 0 for degree of thrombocytopenia since nadir was <10k, 0 for absent thrombosis, 1 for possible other causes); thus, she had low probability for HIT. Subsequently, heparin-PF4 antibodies were found to be negative.
Clopidogrel causes thrombocytopenia by inducing thrombotic thrombocytopenic purpura [Citation5] (TTP) or by inducing an allergic reaction [Citation6]. Neither option was applicable, since there were no signs of microangiopathic hemolytic anemia (normal bilirubin, no schistocytes on the blood smear), and the patient and medical staff reported no symptoms of allergy.
Disseminated intravascular coagulation (DIC) is another likely etiology in this case. DIC is known as consumption coagulopathy; it is an acute life-threatening emergency. It causes a systemic pathologic activation of the coagulation system leading to an extensive formation of thrombi, which in turn leads to consumption of pro- and anticoagulation factors. It also involves fibrinolysis and excessive bleeding. The diagnosis is based on clinical and laboratory criteria. Clinically, the patient should have an underlying condition known to be associated with DIC, either infectious or non-infectious, and present with generalized bleeding and unexplained thrombosis. Laboratory findings include thrombocytopenia, low fibrinogen, elevated prothrombin time (PT), activated partial thromboplastin time (PTT), and D-dimer (a marker of fibrin degradation). These tests should not be taken in isolation. A scoring system was developed by the International Society of Thrombosis and Haemostasis; it includes low platelet count and fibrin-related marker, prolonged PT, and low fibrinogen level. A score greater than or equal to five is compatible with overt DIC[Citation7]. We could not calculate the score in our patient since the D-dimer was not performed. However, it was unlikely, since fibrinogen, international normalized ratio (INR), and PTT were normal ().
As for eptifibatide, a Gp IIb/IIIa receptor antagonist, the first clinical trial of 4,722 patients revealed no difference in the incidence of thrombocytopenia between eptifibatide and the control groups. However, more cases of profound thrombocytopenia (platelet count <20,000) were noticed in the eptifibatide-treated patients than in the placebo group, although the absolute number of patients was very small (nine patients in the eptifibatide-treated group and two in the placebo group). In addition, bleeding was more common in patients treated with eptifibatide compared to placebo. Most of these cases were mild and occurred at the femoral access site [Citation8]. Subsequent case reports had shown that eptifibatide is associated with thrombocytopenia, although the exact mechanism is still under debate. Multiple theories have been discussed.
One case report describes acute thrombocytopenia occurring after a second exposure to eptifibatide[Citation9]. Examination of the bone marrow 4 days after developing the profound thrombocytopenia revealed a predominance of early megakaryocyte stages. An abrupt increase in platelet count was described following platelet transfusion. Nonetheless, it can be hypothesized that the destruction of megakaryocytes had occurred several days earlier while exposed to the drug. Had a bone marrow biopsy been performed at that time, it might have shown absent megakaryocytes in the bone marrow. These findings can be explained by impaired megakaryocytopoiesis complicating anti-GP IIb/IIIa antibody-mediated immune thrombocytopenia [Citation9]. To reproduce the event, Greinacher et al. cultured megakaryocytes in the presence of eptifibatide and the patient’s serum, and increased cell death occurred. In our case, we did not have the chance to monitor the platelet since death occurred 24 hours after thrombocytopenia and eptifibatide administration, but the acute onset of thrombocytopenia makes this theory in our patient less likely.
Another theory is the immune-mediated destruction of circulating platelets via immunoglobulin G (IgG) antibodies that can be naturally present in the patient’s serum even without prior drug exposure. These antibodies target GPIIb/IIIa complexes and are eptifibatide-dependent [Citation10]. The acute development of thrombocytopenia in our case makes this theory more likely; however, the appropriate response to transfusion makes this etiology less likely, unless the activation of these antibodies is eptifibatide dependent because the medication was discontinued before the transfusion. We were unable to monitor the platelet count further to see if it was reversible following eptifibatide discontinuation.
4. Conclusion
Thrombocytopenia in the critical care setting is a frequent occurrence that may be attributed to various etiologies including sepsis, DIC, HIT, TTP, and drug induced. Although eptifibatide may play an important role in certain patients undergoing percutaneous intervention, its ability to cause profound acute thrombocytopenia should not be overlooked. The exact mechanism remains unclear and is under debate. Multiple theories have been formulated. The two main theories are immune destruction of megakaryocytes in the bone marrow and immune destruction of circulating platelets. Our case supports the former theory since there was an acute drop in platelet count following eptifibatide administration. Although the adequate response to platelet transfusion may make this theory less likely, it can be explained by the prerequisite of eptifibatide presence for the activation of these antibodies. Eptifibatide had already been discontinued when she received the platelet transfusion. Our case is not the first to report acute, severe thrombocytopenia with the administration of eptifibatide, but it adds another case to the medical literature. Further research is needed to determine which patients are at high risk for developing profound thrombocytopenia following eptifibatide administration. In summary, it is vital to monitor the platelet count after initiation of eptifibatide, regardless of whether it is the patient’s first exposure, to monitor for this potentially life-threatening complication.
Consent
Informed consent was obtained by the patient’s health care proxy prior to the drafting of this manuscript.
Disclosure statement
The authors have no conflicts of interest to declare.
References
- Phillips DR, Scarborough RM. Clinical pharmacology of eptifibatide. Am J Cardiol. 1997;80(4A):11B–20B. Available from: https://pubmed.ncbi.nlm.nih.gov/9291241/
- Khurana D, Deoke SA. Thrombocytopenia in critically ill patients: clinical and laboratorial behavior and its correlation with short-term outcome during hospitalization. Indian J Crit Care Med. 2017;21(12):861–864. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5752797/
- Elgohary TS, Zaghla HE, Azab AM, et al. Role of thrombocytopenia as an independent prognostic marker in the critically ill patients with multiorganfailure. Med J Cairo Univ. 2011;79. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5752797/
- Arepally GM. Heparin-induced thrombocytopenia. Blood. 2017;129(21):2864–2872. Available from: https://ashpublications.org/blood/article/129/21/2864/36268/Heparin-induced-thrombocytopenia
- Zakarija A, Kwaan HC, Moake JL, et al. Ticlopidine- and clopidogrel-associated thrombotic thrombocytopenic purpura (TTP): review of clinical, laboratory, epidemiological, and pharmacovigilance findings (1989-2008). Kidney Int Suppl. 2009;75(112):S20–4. Available from: https://pubmed.ncbi.nlm.nih.gov/19180126/
- Guo YL, Li JJ, Yuan JQ, et al. Profound thrombocytopenia induced by clopidogrel with a prior history of long-term safe administration. World J Cardiol. 2010;2(6):160–162. Available from: https://pubmed.ncbi.nlm.nih.gov/21160734/
- Taylor F, Toh C, Hoots K, et al. Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemost. 2001;86:1327–1330.
- Investigators, P. G. I. I. i. U. A. R. S. U. I. T. P. T. Inhibition of platelet glycoprotein IIb/IIIa with eptifibatide in patients with acute coronary syndromes. N Engl J Med. 1998;339(7):436–443. Available from: https://pubmed.ncbi.nlm.nih.gov/9705684/
- Greinacher A, Fuerll B, Zinke H, et al. Megakaryocyte impairment by eptifibatide-induced antibodies causes prolonged thrombocytopenia. Blood. 2009;114(6):1250–1253. Available from: https://pubmed.ncbi.nlm.nih.gov/19429867/
- Bougie DW, Wilker PR, Wuitschick ED, et al. Acute thrombocytopenia after treatment with tirofiban or eptifibatide is associated with antibodies specific for ligand-occupied GPIIb/IIIa. Blood. 2002;100(6):2071–2076. Available from: https://pubmed.ncbi.nlm.nih.gov/12200368/