ABSTRACT
Mitral valve infective endocarditis, without aortic involvement, is a rare cause of complete heart block. It is thought that infections placed close to the conductive system of the heart may drive a conduction block. We found six cases in the literature, via searching PubMed, of mitral valve infective endocarditis with complete heart block and no aortic involvement. We report a case of complete heart block with a junctional escape rhythm in a patient with a Staphylococcus Aureus vegetation on a native mitral valve only.
1. Introduction
Infections of the heart valves, particularly the aortic valve, are sometimes placed close to the conduction system of the heart to cause the complication of complete heart block. Mitral valve infective endocarditis, without aortic involvement, is rare in the literature as a source of complete heart block. We report a rare case of complete heart block with a junctional escape rhythm in a patient with a vegetation on a native mitral valve. We found six cases, via searching PubMed, of mitral valve infective endocarditis with complete heart block, without aortic involvement. As late as 2005, the literature had reported no previous cases of mitral valve infective endocarditis with complete heart block [Citation1].
2. Case presentation
We present a 55-year-old male patient with a past medical history of end-stage renal disease (‘ESRD’), hypertension, asthma, and intravenous drug use, who presented to our facility emergency department with bleeding from his left arm arteriovenous (‘AV’) fistula site and complete heart block (‘CHB’). He had missed two sessions of hemodialysis prior to presentation. His known active symptoms included left arm pain and bleeding. The patient denied chest pain, dyspnea, or palpitations in the emergency department. His entire review of systems disclosed no other active symptomatology, although he was a poor historian. On admission, his blood pressure was elevated to 189/110 mm Hg and his pulse was 58 beats per minute (‘BPM’). His lungs were clear to auscultation. His cardiac examination was unremarkable for any rubs, murmurs, or gallops. The remainder of the physical examination was unremarkable. His potassium was elevated to 6.5 mmol/L. However, the remainder of his blood work including thyroid-stimulating hormone (‘TSH’) and lyme serology were unremarkable.
The patient’s hyperkalemia was corrected, and he had an emergent dialysis session. Despite addressing other potential causes of heart block, the patient’s complete heart block persisted. The bleeding left arm AV fistula was corrected surgically with an interposition graft.
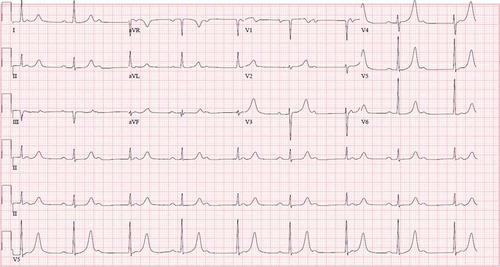
An electrocardiogram showed complete heart block with a junctional escape rhythm in the 50s bpm (). Also, during his hospital stay, the patient developed different arrhythmias including intermittent atrial flutter with 3:1 block and accelerated junctional escape rhythm.
A transthoracic echocardiogram (‘TTE’) was performed which showed a normal left ventricular chamber. The global left ventricular wall motion and contractility were within normal limits. The mitral valve leaflets were normal in structure. There was a mild to moderate mitral regurgitation. A mass consistent with a vegetation was visualized on the anterior leaflet of the mitral valve in close proximity to the aortomitral cushion. The parasternal long-axis view failed to show any abscess formation around the aortic root.
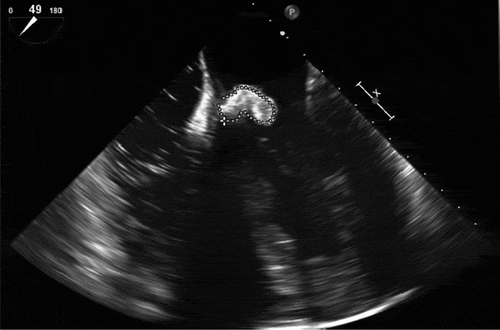
A later transesophageal echocardiogram (‘TEE’) showed moderate to severe mitral regurgitation with a posteriorly directed jet and a bilobular vegetation 1.42 cm x 0.99 cm on the proximal portion of the anterior mitral valve leaflet in close proximity to the aortic root. No abscess was visualized on the mitral valve (). No vegetations were observed on the tricuspid or aortic valve. Also, the TEE failed to show any abscess cavity formation close to the aortic root.
Blood cultures were positive for methicillin-sensitive staphylococcus aureus (‘MSSA’). The patient was treated with intravenous Cefazolin on hemodialysis days for his MSSA bacteremia. After his definitive mitral valve surgery, he was continued on a course of intravenous cefazolin on dialysis days for 4 weeks as per the infectious diseases team.
Operative replacement of a prosthetic mitral valve and tricuspid valve annuloplasty was performed. In the operating room, the mitral valve was inspected and seen as heavily diseased with fibrinous debris on both leaflets and heaping of tissues. The tricuspid valve annulus was dilated.
On the first day postoperatively of his mitral valve repair surgery, he continued to have complete heart block with the same stable underlying junctional escape rhythm. He was transvenously paced by the cardiology team. His complete heart block never resolved, and a dual-chamber permanent pacemaker was inserted 1 week after his mitral valve surgery.
The patient clinically denied chest pain, dyspnea, or other symptoms at the time of discharge. He was discharged for further outpatient follow-up with a schedule of hemodialysis to complete his course of 4 weeks of intravenous cefazolin on dialysis days.
3. Discussion and literature review
Infective endocarditis is a disease associated with high morbidity and mortality. A multi-week course of antibiotic treatment with or without surgical intervention is indicated [Citation2]. Mitral valve endocarditis is uncommon on its own as a cause of complete heart block. A few cases have been reported. They suggest that abscesses or extension of infection beyond the valve with damage to the nearby conduction system are possible mechanisms driving heart block. Furthermore, there seems to be a pattern of anterior mitral valve leaflet involvement and staphylococcus aureus infection in many of these cases.
For example, the authors of a 2005 report claimed complete heart block had not been reported with mitral valve infective endocarditis before their case. In that case, a large mitral valve annular abscess with vegetations on the mitral leaflets, with no aortic involvement and progression from first-degree heart block to complete heart block, was found in a 66 year old female with an abscess at a fistula site on dialysis. In that case, debridement and surgical replacement of the mitral valve with pacemaker insertion were used. Some similarities to our case included the history of dialysis and an infection at an AV fistula site. However, unlike our case, there was evidence of a full mitral valve annulus abscess.
In another 2006 case, the authors claimed there were no reports in the literature of previous cases of mitral valve infective endocarditis presenting with high-grade atrioventricular block. In that case, a 37 year old woman with renal insufficiency and MSSA bacteremia was found to have a 1.4 cm vegetation extending from the anterior leaflet of the mitral valve through the fibrous trigone into the right atrium, with a second 0.9 cm vegetation on the posterior leaflet of the mitral valve [Citation3]. She also had a complete heart block. Her mitral valve was replaced, and a pacemaker was implanted. In this case, like ours, the patient had presented with abnormal electrolytes and renal insufficiency and some involvement of the anterior mitral valve leaflet.
Another case in 2010, similar to ours, was reported of MSSA mitral valve infective endocarditis on the anterior mitral leaflet that presented with fever and a new complete atrioventricular block [Citation4]. The authors canvassed the literature to report that abscess destruction or inflammation and edema extension might affect the nearby conduction system.
Furthermore, a 2012 case in the Portuguese literature reported a 62-year-old male admitted for atrial fibrillation and complete heart block due to methicillin-resistant staphylococcus aureus (‘MRSA’) mitral native valve endocarditis predominately on the anterior mitral leaflet with vegetations also on the septal tricuspid leaflet [Citation5]. The authors proposed the mechanism of complete heart block was bacteremia leading to mitral valve infective endocarditis and then an extension to the mitral-aortic junction and tricuspid valve, damaging the conduction system.
In 2016, a case of mitral valve infective endocarditis with initial first-degree heart block evolving into complete heart block during the inpatient hospital stay and eventual death was reported due to Streptococcus agalactiae [Citation6]. The case involved a large 2 × 1 cm native mitral valve vegetation with a perforated posterior mitral leaflet, a tricuspid septal leaflet vegetation, interventricular septum extension, and extensive necrosis of the atrioventricular node based on postmortem exam. Unlike our case, a mechanism of perivalvular extension and documented atrioventricular node necrosis was the obvious mechanism of complete heart block.
A case similar to ours occurred in 2019, wherein a 19-year-old female, without a past medical history, presented with anterior mitral valve Staphylococcus aureus infective endocarditis with complete heart block and a junctional rhythm [Citation7]. Despite the insertion of a prosthetic valve, the patient died soon after discharge, however. Once again anterior mitral valve leaflet involvement with Staphylococcus aureus was found.
The literature suggests mitral valve infective endocarditis, without aortic involvement, is an extremely rare cause of complete heart block, even on top of the rarity of infective endocarditis driven complete heart block. The few case reports show a tendency for anterior mitral valve leaflet involvement, often Staphylococcus aureus infection, and sometimes renal comorbidities. The cases have ranged from vegetations on the mitral valve, to large abscesses to fully documented atrioventricular node necrosis. Presumably, damage to the nearby conduction system near the mitral valve, particularly near the anterior mitral valve, causes complete heart block, possibly through some extension of infection or inflammation.
4. Conclusion
Mitral valve infective endocarditis can present with complete heart block without echocardiographic evidence of aortic involvement. Anterior mitral leaflet involvement is often found in cases of mitral valve infective endocarditis with complete heart block. Continuous antibiotic treatment, with the replacement of line sites, surgical correction, and pacemaker insertion at the end of a protracted hospital stay can result in positive outcomes as with this case. Intermittent atrial flutter superimposed on the background of complete heart block can complicate treatment plans. In cases of complete heart block and infective endocarditis, mitral valve infective endocarditis without aortic involvement can remain in consideration as a rare but possible cause of this arrhythmia.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Javaid M, Awasthi A, Fink G, et al. Complete heart block associated with mitral annular abscess. Mayo Clin Proc. 2005;80(11):1531–1532.
- Baddour Larry M, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications. Circulation. 2015;132(15):1435–1486.
- Porter TR, Airey K, Quader M, et al. Mitral valve endocarditis presenting as complete heart block. Tex Heart Inst J. 2006;33(1):100–101.
- Kitkungvan D, Suri J, Spodick D, et al. Complete atrioventricular block: a rare presentation of mitral valve endocarditis. J Invasive Cardiol. 2010;22(1):E1–2.
- Sousa A, Lebreiro A, Sousa C, et al. An atypical presentation of infective endocarditis. Rev Port Cardiol. 2012;31(12):829–832.
- Arai M, Nagashima K, Kato M, et al. Complete atrioventricular block complicating mitral infective endocarditis caused by streptococcus agalactiae. Am J Case Rep. 2016;17:650–654.
- Cap M, Erdoğan E. Acute mitral valve endocarditis complicated by complete atrioventricular block, junctional escape rhythm, and skin manifestations. Anatol J Cardiol. 2019;22(5):5012.