ABSTRACT
Eosinophilic pneumonia is a category of lung diseases characterized by an increased number of eosinophils in alveolar spaces and interstitium. Acute cases are often caused by fungal infections, parasites, drugs or toxins and can present with respiratory failure. Daptomycin has been identified as one of the rare causes of acute eosinophilic pneumonia. We describe a case of an elderly man on daptomycin for MRSA endocarditis treatment who presented to the hospital with fevers and dyspnea within two weeks of daptomycin initiation. As an inpatient, he developed an increasing oxygen requirement necessitating intensive care unit management. Daptomycin cessation improved his symptoms and he was placed on a steroid taper. These findings suggested a diagnosis of daptomycin-induced eosinophilic pneumonia. However, the patient deteriorated and eventually passed away despite resuscitative efforts. This case highlights the importance of prompt identification of eosinophilic pneumonia, its potential severity and the need for more exploration regarding the timing of corticosteroid taper. This in turn will inform more effective approaches to this condition in the future.
1. Introduction
Eosinophilic pneumonia is a category of lung diseases characterized by eosinophil infiltration of alveolar spaces and lung interstitium[Citation1]. Acute cases are rare and thus the epidemiology is not well understood [Citation2,Citation3]. In addition, there are numerous causes of eosinophilic pneumonia including fungal infection, parasites, drugs and toxins [Citation1,Citation2,Citation4]. A few studies have identified daptomycin as a cause of acute eosinophilic pneumonia (AEP) [Citation4–7, Citation11].
Daptomycin is a cyclic lipopeptide antibiotic that is FDA approved for use in the treatment of skin and bloodstream infections [Citation5,Citation8]. It exhibits bactericidal activity against gram-positive bacteria including methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant enterococcus (VRE) [Citation4,Citation8]. One of the most common adverse effects associated with daptomycin is skeletal myopathy characterized by an increase in creatinine kinase [Citation9]. However, daptomycin has also been identified as a rare cause of eosinophilic pneumonia.
We describe a case of an elderly man on daptomycin for MRSA endocarditis treatment who presented to the hospital with fevers and dyspnea within 2 weeks of initiation. The purpose of this paper is to provide more data about this clinical disease as well as highlight potential variations in presentation and management.
2. Case presentation
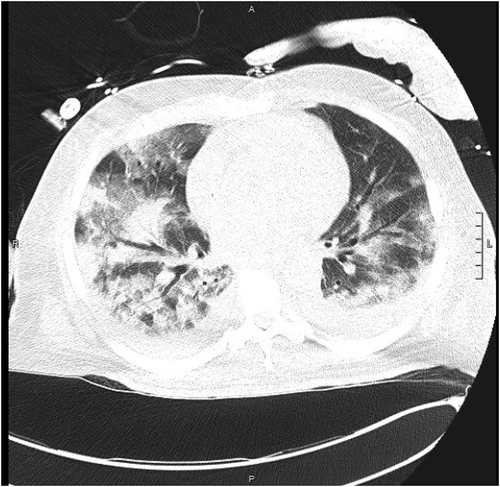
A 71-year-old African American man with a history of obesity, hypertension, hyperlipidemia, diabetes and undergoing treatment with 800 mg IV daptomycin (completed two of six weeks of treatment) for aortic valve MRSA endocarditis presented from subacute rehab after nursing staff noted his acute dyspnea for one day. In the emergency department, his oxygen saturation was 83% on room air. The rest of his vital signs were normal. Aside from some increased work of breathing and diffuse rhonchi on lung exam, his physical examination was non-focal and unremarkable. Initial laboratory exam revealed leukocytosis of 15.3k with 0.4% immature granulocytes, making the white count more likely secondary to stress than infection. Besides, eosinophils were as high as 6.3%. Hemoglobin, hematocrit, platelet count and complete metabolic panel were unremarkable. An arterial blood gas analysis on room air reflected a primary respiratory alkalosis (ABG: pH 7.52, PaCO2 31, PO2 80, HCO3 25, SaO2 98). His chest x-ray showed bilateral pulmonary infiltrates (). CT chest without contrast confirmed the presence of bilateral patchy airspace disease and ground-glass infiltrates consistent with pneumonia or inflammatory lung disease but not typical of classic congestive heart failure (). It was noted that in his prior diagnosis of MRSA endocarditis, the patient was discharged on only Daptomycin. With these data, the initial working diagnosis was between an atypical pneumonia and an inflammatory process perhaps from daptomycin toxicity. Infectious disease was consulted who also suspected daptomycin induced lung injury thus, daptomycin was stopped. He received non-invasive ventilation and ceftaroline for pneumonia and endocarditis.
Figure 1. Erect anteroposterior (AP) chest x-ray taken with a portable (port) x-ray machine upon admission to the emergency department showing bilateral alveolar infiltrates and diffuse bilateral ground glass opacities. R = right side
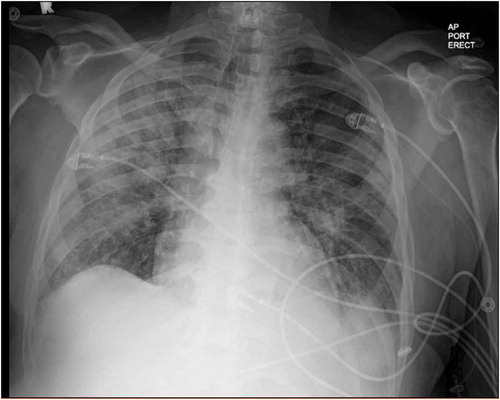
Figure 2. Axial section CT non-enhanced (lung window), section 60, obtained in the emergency department demonstrating ground glass infiltration. L = Left side, A = anterior, P = posterior, scale = 1cm.
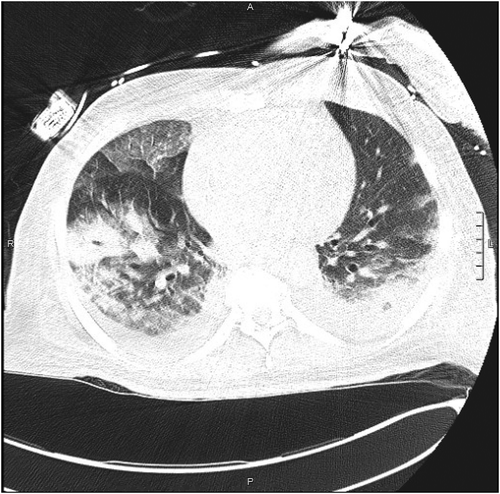
Figure 3. Axial section CT non-enhanced (lung window), section 55, obtained in the emergency department demonstrating further ground glass infiltration. L = Left side, A = anterior, P = posterior, scale = 1cm
Shortly after admission, his oxygenation remained poor despite escalating oxygen support (high flow oxygen 50 L, 80% then BiPAP 18/10 with FiO2 100%), he was transferred to the intensive care unit (ICU) for impending intubation. The next day, recognizing that his pulmonary status was tenuous and rationalizing that this was likely secondary to inflammation, the primary care team started him on methylprednisolone 60 mg q6h. After this, his respiratory rate slowed from 30 to 16–20 breaths per minute. His oxygen requirement decreased and his work of breathing improved.
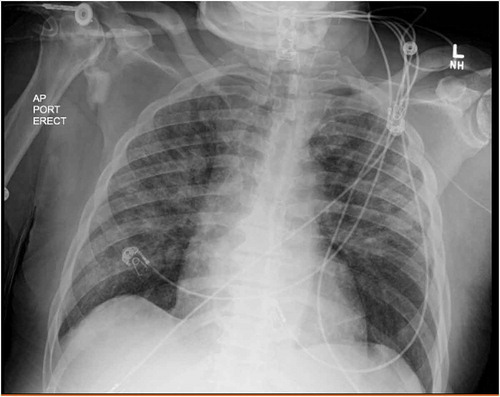
Over the next 2 days, he was on lower ventilatory support (BiPAP 16/10 with FiO2 down to 0.6) and his oxygen saturations were 99% allowing his transition to a high-flow system. His chest x-ray showed >75% clearing of infiltrate compared to the initial film (). His clinical improvement in the absence of daptomycin and the presence of high-dose corticosteroid supported the hypothesis that his pulmonary process was driven by an inflammatory response likely due to the daptomycin.
Figure 4. Erect anteroposterior (AP) chest x-ray taken with a portable (port) x-ray machine one day after daptomycin was discontinued showing decreased bilateral alveolar infiltrates compared to Figure 1. L = left side
After five days in the ICU, the patient was transitioned to nasal cannula, planned for a 20-day prednisone taper from 40 mg and transferred to a step-down unit. Unfortunately, his improvement was short-lived, and his oxygen need increased again requiring BiPAP (70 L, 90%) by day 10 in the hospital. He was given a dose of methylprednisolone 60 mg IV and transferred back to the ICU. At this point in his hospitalization, the working diagnosis was either an ongoing partially treated drug-induced lung injury on a lower steroid dose or an alternative steroid-responsive process such as vasculitis or non-vasculitis inflammatory lung disease. Of note, sputum cultures were ordered but never obtained throughout his hospitalization because he never produced an adequate amount of sputum He was placed on methylprednisolone 60 mg IV daily and immunological workup including ANA, anti-dsDNA, anti-Smith antibody, anti-histone antibody, p-ANCA, c-ANCA, anti-GBM antibody, RF, anti-CCP were sent which were all normal. He developed respiratory failure. Unfortunately, during intubation, the patient developed a pulseless electrical activity (PEA) arrest due to hypoxia with a return of spontaneous circulation in 10 minutes. He suffered three more PEA arrests which are thought to be secondary to severe hypoxic hypercapnic respiratory failure and metabolic acidosis. After the 4th arrest, he was pronounced dead.
3. Discussion
This paper highlights a case of daptomycin-induced eosinophilic pneumonia (DAP-Induced EP) in a 71-year-old male. Eosinophilic pneumonia can be further subdivided into primary or secondary disorders, where a primary is typically idiopathic and secondary is due to the presence of an identifiable cause [Citation1]. Acute eosinophilic pneumonia is a secondary disorder that can be caused by drugs, toxins, parasites and fungal infections [Citation1,Citation2,Citation4]. In particular, drug-induced AEP has been reported to be associated with the use of daptomycin, mesalamine, sulfasalazine and minocycline. However, this condition is quite rare and thus the epidemiology is poorly understood. One large-scale literature review exploring drug-induced AEP found 228 cases reported between 1990 and 2017, with only 137 cases meeting their inclusion criteria. The demographics for these cases ranged from 18 to 82 years of age and affected males and females equally. Of these patients, 21% had an acute hypoxemic respiratory failure of which 19.8% required mechanical ventilation thus highlighting the severity of this condition. This study also found that daptomycin use accounted for one of the highest numbers of drug-induced AEP cases which emphasizes the need for a better understanding of this correlation [Citation10,Citation11].
To effectively assess this condition, it is important to understand the pathophysiology. One study proposed that the detection of antigens by alveolar macrophages leads to the recruitment of T-helper 2 lymphocytes and the release of interleukin 5. The function of interleukin 5 is to induce the production of eosinophils and migration to the lung. Eotaxin, an eosinophil chemoattractant, is produced by alveolar macrophages and other cells in the lungs. This substance further induces an accumulation of eosinophils. It is hypothesized that daptomycin is retained in the pulmonary surfactant and when concentrations become high enough, it can lead to injury of the surrounding tissues. This injury will then activate alveolar macrophages and initiate the process of eosinophil accumulation [Citation12].
Acute cases of eosinophilic pneumonia often present with a rapid onset of dyspnea, non-productive cough and fever [Citation2,Citation13]. On physical exam, patients present with tachypnea, diffuse inspiratory crackles and rhonchi [Citation2]. Imaging of AEP usually shows bilateral reticular ground-glass opacities that spread as the disease progresses [Citation10]. In addition, patients with eosinophilic pneumonia present with eosinophilia in the blood (eosinophilic count >500 × 109 cells/L) and bronchoalveolar lavage fluid (>5% eosinophils in white blood cell differential count) [Citation10]. Disease severity can vary from a mild disease to severe respiratory failure [Citation13] however factors related to increased severity are poorly understood.
The diagnosis of daptomycin-induced eosinophilic pneumonia is typically made after meeting several proposed criteria including concurrent exposure to daptomycin, fever, dyspnea with increased oxygen requirement or requirement of mechanical ventilation, new infiltrates on chest x-ray or CT scan, bronchoalveolar lavage with >25% eosinophils and improvement after daptomycin withdrawal [Citation5,Citation6]. Higashi et al. (2018) also explored whether skin testing could be useful in detecting a potential risk of developing daptomycin-induced eosinophilic pneumonia. However, they found no correlation between a positive skin test and susceptibility to developing DAP-induced EP [Citation4]. Our patient met all but one of the above criteria as a bronchoalveolar lavage was not done after considering the risks and benefits. For their literature review, Kim et al. (2012) created inclusion criteria to diagnose DAP-induced EP as definite, probable, possible or unlikely[Citation6]. Using that criteria, our patient falls under probable DAP-induced EP due to the presence of peripheral eosinophilia.
Current treatments of DAP-induced EP include removal of daptomycin and treatment with corticosteroids. Uppal et al. (2016) looked at 35 cases of DAP-induced EP and found that a majority of these cases showed improvement or recovery after the removal of daptomycin and subsequent corticosteroid treatment. More specifically, patients typically saw improvement one to seven days after daptomycin removal [Citation7]. In the case of our patient, the improvement was seen 24 h after the removal of daptomycin and continued to improve until day 10 when the patient began having an increased oxygen requirement. Thus, it is important to closely review this case and reflect upon what improvements could have been made to allow for a more favorable outcome. Despite the benefit of corticosteroid treatment in AEP patients, one case did show recurrence of eosinophilic pneumonia following steroid taper after which the patient required low dose steroid treatment for 2 years [Citation7,Citation14]. Unfortunately, due to the rarity of this disease, there is no well-established guideline for corticosteroid treatment in DAP-induced EP. However, the typical regimen employed in other patients includes IV methylprednisolone 60–125 mg every six hours with conversion to PO prednisone 40–60 mg daily and taper over two to six weeks [Citation7]. Our patient was given IV methylprednisolone and was planned for a two-week prednisone taper. However, perhaps a longer taper would have been more beneficial given the patient’s age and severity of respiratory failure as indicated by oxygen requirement. Future studies could explore the recommended prednisone taper based on the severity of the patient’s case.
Due to the rarity of drug-induced EP, many aspects of it are poorly understood. As mentioned previously, many patients do show improvement or recovery after treatment [Citation7]. However, no studies have looked at the long-term impacts and thus, there is a poor understanding of the potential complications of this condition. More research on this subject could inform more holistic approaches to treatment and allow for better patient outcomes in the long term.
4. Conclusion
This study highlights the importance of prompt identification of eosinophilic pneumonia. Based on the literature, this disease is quite severe due to the potential for the development of acute respiratory failure. However, research has shown that removal of the offending agent and appropriate treatment with corticosteroids can lead to recovery [Citation6,Citation7]. Thus, a set of diagnostic criteria for eosinophilic pneumonia should be established to help lead to a prompt diagnosis. Future research can explore potential risk factors and complications associated with drug-induced AEP. Finally, a thorough exploration of corticosteroid treatment variations can inform more effective approaches to this condition in the future.
Disclaimer
The views expressed in this article are our own and not an official position of the institution.
Acknowledgments
No acknowledgments.
Disclosure statement
No potential conflict of interest was reported by the authors.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Cottin V. Eosinophilic lung diseases. Clin Chest Med. [Internet]. 2016;37(3):535–556.
- De Giacomi F, Vassallo R, Yi ES, et al. Acute eosinophilic pneumonia. Am J Respir Crit Care Med. 2018;197(6):728–736.
- Rhee CK, Min KH, Yim NY, et al. Clinical characteristics and corticosteroid treatment of acute eosinophilic pneumonia. Eur Respir J. 2013;41(2):402–409.
- Higashi Y, Nakamura S, Tsuji Y, et al. Daptomycin-induced eosinophilic pneumonia and a review of the published literature. Intern Med. 2018;57(2):253–258.
- US Food and Drug Administration. FDA drug safety communication: eosinophilic pneumonia associated with the use of Cubicin (daptomycin). http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm220273.htm
- Kim PW, Sorbello AF, Wassel RT, et al. Eosinophilic pneumonia in patients treated with daptomycin: review of the literature and US FDA adverse event reporting system reports. Drug Saf. 2012;35:447–457.
- Uppal P, LaPlante KL, Gaitanis MM, et al. Daptomycin-induced eosinophilic pneumonia - a systematic review. Antimicrob Resist Infect Control. 2016;5(55). DOI:10.1186/s13756-016-0158-8
- Heidary M, Khosravi AD, Khoshnood S, et al. Daptomycin. J Antimicrob Chemother. 2018;73(1):1–11.
- Package insert. Cubicin (daptomycin) injection, cubist pharmaceuticals, Lexington, MA, November 2010.
- Bartal C, Sagy I, Barski L. Drug-induced eosinophilic pneumonia. Medicine. 2018;97:4.
- Patel JJ, Antony A, Herrera M, et al. Daptomycin-induced acute eosinophilic pneumonia. Wis Med J. 2014;113(5):199–201.
- Silverman JA, Mortin LI, Vanpraagh AD, et al. Inhibition of daptomycin by pulmonary surfactant: in vitro modeling and clinical impact. J Infect Dis. 2005;191:2149–2152.
- Suzuki Y, Suda T. Eosinophilic pneumonia: a review of the previous literature, causes, diagnosis, and management. Allergol Int. Internet. 2019;68(4):413–419.
- Lal Y, Assimacopoulos AP. Two cases of daptomycin-induced eosinophilic pneumonia and chronic pneumonitis. Clin Infect Dis. 2010;50(5):737–740.
