KEYWORDS:
1. Background
Chronic hepatitis C (CHC) is associated with a higher risk of developing type 2 diabetes mellitus (DM)[Citation1]. In patients with preexisting type 2 DM, CHC often worsens glycemic control[Citation1]. With direct-acting antivirals (DAAs)-based treatment regimens for CHC, nearly all patients achieve sustained virologic response (SVR)[Citation2]. There is limited literature demonstrating improvement in the glycemic index of patients with DM following the eradication of hepatitis C virus (HCV) with DAA[Citation1]. Thus, it is reasonable to hypothesize that early treatment of CHC and optimal glycemic control in these patients could prevent chronic complications of diabetes and worsening of liver disease. We performed a retrospective cohort study examining whether HCV eradication with DAAs leads to improved glycemic index in patients with DM and the feasibility of safely and successfully offering such care at a primary care physician/providers (PCP) office.
2. Methods
We performed a retrospective chart review over 18 months of CHC patients with preexisting DM who had achieved SVR using DAAs. Baseline demographics and disease characteristics were recorded, including age, gender, date of diagnosis of HCV infection, change in body weight, and physical activity. The Vibration Controlled Transient Elastography (VCTE) (Fibroscan®) staging before treatment was noted. Change in HbA1c and antidiabetic medications before and after achieving SVR was also recorded. The study was approved by the Institutional Review Board (IRB).
3. Results
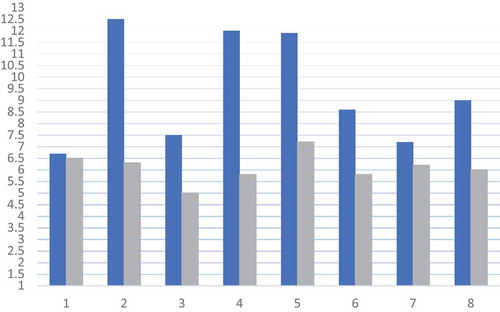
Of 180 patients with CHC, 12 had preexisting DM. Eight of 12 patients achieved SVR. All patients were men with a mean age of 58.4 years. VCTE before treatment initiation showed Metavir stage F4 in 75% of patients (). All 12 patients had DM for over 10 years with at least one micro- or macrovascular complications. The mean HbA1c decreased from 9.4% to 6.1% after SVR (). Following SVR, 37% patients had significant reduction in antidiabetic medication dose, with one patient going off all anti-hyperglycemic medications (). No clinical or biochemical hypoglycemic events were documented. There was no significant change in body weight.
Table 1. Change in HbA1c and antidiabetic medications post SVR with DAAs in CHC patients
4. Discussion
Our study demonstrated a decrease in HbA1c levels among all patients with SVR. The decrease in HbA1c was more pronounced in patients with a higher baseline HbA1c value. The mechanism on how SVR affects the glycemic index is not clear but it is mostly due to significant decrease in the homeostasis model assessment of insulin resistance (HOMA-IR) index based on fasting glucose and insulin levels[Citation3].
The USA Preventative Services Task Force (USPSTF) recommends screening all adults above the age of 18 for CHC[Citation4]. PCPs play a crucial role in screening, enabling more patients to be diagnosed and treated[Citation5]. With current regimens being a lot less complicated, PCPs can appropriately provide treatment for these patients, minimizing care costs, referrals, and fewer patients lost to follow up. We emphasize the importance of a multidisciplinary team approach for treating CHC led by the PCP as the team leader.
One challenge acknowledged by the American Association for the Study of Liver Diseases (AASLD) is accurate staging of fibrosis and need for long-term follow-up in stage 3 or 4 fibrosis as these patients need hepatocellular carcinoma (HCC) surveillance[Citation6]. We used VCTE for these staging prerequisites.
Here, we propose a simple protocol that can be used at a PCP’s office to treat CHC successfully:
Step 1. Confirm the chronicity of CHC, obtain HCV RNA 6 months apart.
Step 2. Fibrosis staging, VCTE, or laboratory panels [Citation6]. Refer to a specialist as appropriate.
Step 3. Baseline imaging study, to rule out cirrhosis or HCC.
Step 4. Start DAA therapy and repeat HCV RNA as recommended. Assess end of treatment response and document SVR.
In conclusion, there is an association of CHC in exacerbating metabolic syndrome and DM resulting from mechanisms that are not clearly understood. Although large randomized control studies are required to establish this effect firmly, our single-center study findings support timely screening, diagnosis, staging of fibrosis, and prompt initiation of DAAs for CHC at the PCP office with improved patient outcomes, especially in preexisting DM-2. Most importantly, access to care is significantly broadened because a PCP can care for the larger majority of these patients and need to refer to a subspecialist only in a smaller minority.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Hum J, Jou JH, Green PK, et al. Improvement in glycemic control of type 2 diabetes after successful treatment of hepatitis C virus. Diabetes Care. 2017;40(9):1173–1180.
- Luo A, Xu P, Wang J, et al. Efficacy and safety of direct-acting antiviral therapy for chronic hepatitis C genotype 6: A meta-analysis. Medicine (Baltimore). 2019;98(20):e15626.
- Kawaguchi T, Ide T, Taniguchi E, et al. Clearance of HCV improves insulin resistance, beta-cell function, and hepatic expression of insulin receptor substrate 1 and 2. Am J Gastroenterol. 2007;102(3):570–576.
- Rosenberg ES, Barocas JA. USPSTF’s hepatitis C screening recommendation-a necessary step to tackling an evolving epidemic. JAMA Network Open. 2020;3(3):e200538.
- Tran TT. Hepatitis C: who should treat hepatitis C virus? The role of the primary care provider. Clin Liver Dis. 2018;11(3):66–68.
- Ghany MG, Morgan TR. AASLD-IDSA hepatitis C guidance panel. Hepatitis C guidance 2019 update: American association for the study of liver diseases-infectious diseases society of america recommendations for testing, managing, and treating hepatitis C virus infection. Hepatology. 2020;71(2):686–721.