ABSTRACT
Duodenal ectopic varices (DEV) are an uncommon etiology of upper gastrointestinal bleeding and are associated with high mortality. Both the diagnosis and management of DEV are challenging. Multiple treatment modalities exist including endoscopic guided management (ligation and sclerotherapy), surgical resection, transvenous obliteration and transjugular intrahepatic portosystemic shunt (TIPS), but management depends on the underlying vascular anatomy and underlying pathology. We present a case of a 41-year-old man with a history of an alcohol use disorder, prior splenic vein thrombosis as a complication of pancreatitis who presented with massive gastrointestinal bleeding, and was ultimately diagnosed with distal duodenal ectopic varix, which contained inflow from a medial branch of the superior mesenteric vein and outflow into the left renal vein. He was successfully treated with transjugular portosystemic shunt and coil embolization.
1. Introduction
Gastrointestinal bleeding (GIB) is a significant cause of morbidity and mortality worldwide. Gastrointestinal bleeding from proximal sources (esophagus, stomach and duodenum) has an incidence of 47/100,000 and lower gastrointestinal bleeding has an incidence of 33/100,000 [Citation1]. Ectopic varices are an uncommon source of gastrointestinal bleeding, accounting for only 2–5% of variceal bleeding [Citation2]. Ectopic varices are defined as large portosystemic collateral veins occurring anywhere in the gastrointestinal tract other than the esophagogastric region [Citation3]. Though ectopic varices can occur at numerous sites in the GI tract, 17% of the ectopic varices are located in the duodenum [Citation2]. Duodenal varices are generally associated with portal hypertension, which can be secondary to cirrhosis or extrahepatic venous obstruction [Citation2]. Despite the uncommon occurrence of ectopic varices, they have a four-times higher risk of bleeding than gastroesophageal varices [Citation4], and mortality is as high as 40% [Citation5]. We report a case of massive gastrointestinal bleeding due to distal duodenal ectopic varix successfully treated with interventional radiology guided transcatheter embolization and transjugular intrahepatic portosystemic shunt (TIPS) procedure.
2. Case
A forty-one-year-old man was admitted to our hospital with twelve hours of melena and non-bloody emesis. His medical history was significant for alcohol use disorder, prior acute pancreatitis complicated by splenic vein thrombosis, type II diabetes mellitus, and obstructive sleep apnea. A computerized tomography (CT) of the abdomen seventeen months earlier demonstrated splenic vein occlusion vs. marked narrowing with extensive collateral circulation in the upper abdomen. Prior to admission, he had been taking 1600 mg of ibuprofen daily and drinking six beers daily. On exam the patient was tachycardic (132 bpm), blood pressure was 120/70. He was pale, ill appearing and had mild epigastric tenderness on palpation. There were no stigmata of liver disease. On admission the hemoglobin and hematocrit were 8.4 g/dL and 25% respectively. The remaining laboratory results were significant for elevated lactate at 3.7 U/L, INR 1.3, PT 15.7 seconds, PTT 29 seconds, alkaline phosphatase 46 U/L, albumin 3.2 g/dL, total bilirubin 1.5 mg/dL, AST 34 U/L, ALT 30 U/L and platelets 178,000/mm3. He was resuscitated with IV fluids, started on IV proton pump inhibitor, IV octreotide, transfused 1 unit of packed red blood cells (pRBCs), and was admitted to the Medical Intensive Care Unit.
An upper endoscopy was performed on the evening of his admission which demonstrated mildly friable gastric mucosa but did not show any varices or other signs of portal hypertension. His IV pantoprazole and octreotide were stopped at that time. The patient was prepped for a colonoscopy scheduled the following day. Overnight he developed bright red blood per rectum. Despite two additional units of pRBCs, his hemoglobin and hematocrit remained low at 7.1 g/dL and 21.1% respectively. On hospital day (HD) 2, the patient underwent colonoscopy which demonstrated clotted blood throughout the colon and blood streaming down from the terminal ileum, but no bleeding source was identified.
The patient subsequently underwent a CT angiogram of the abdomen and pelvis which demonstrated pericecal-periappendiceal inflammatory changes with mild thickening of the cecal wall, but the angiogram was unable to localize the source of bleeding. The liver edge appeared slightly irregular, but there was no definitive evidence of cirrhosis or obvious signs of portal hypertension, such as ascites. The patient continued to have multiple voluminous episodes of bright red blood per rectum including numerous clots. He remained tachycardic with rates as high as 132 bpm and systolic blood pressure in the 100s. Labs were significant for a hemoglobin of 5.8 g/dL and hematocrit of 16.7%. Due to hemodynamic instability and ongoing bleeding, the patient was transferred to an outside facility for massive transfusion protocol.
Upon arrival at the outside facility massive transfusion protocol was initiated and interventional radiology performed a mesenteric angiogram with the goal of source embolization, however no source of bleeding was identified. Several hours later the patient underwent a CT angiogram of the abdomen and pelvis, which also was unrevealing. Repeat colonoscopy was normal without blood or source of bleeding identified. Due to the ongoing need for transfusion, an exploratory laparotomy was performed with intraoperative push enteroscopy. Enterotomy was performed in the small bowel and no obvious source of bleeding was found. Right hemicolectomy was performed.
Following surgery, the patient continued to pass bright red blood per rectum and required multiple additional pRBC transfusions. On HD 3 interventional radiology performed a transcatheter angiogram and venogram which demonstrated a portal systemic varix with prominent inflow into the mid-left renal vein (). The varix outflow was embolized with multiple 0.35 interlock coils (). After embolization, there was ongoing bleeding, but significantly decreased.
Figure 1. Interventional venogram demonstrating portal systemic varix with inflow from left renal vein and extravasation of contrast into distal duodenum. Annotations as follows: I: IVC, II: Left renal vein, III: left gonadal vein, IV: extravasation of contrast into duodenum, V: Duodenal ectopic varix, Arrow: efferent flow from DEV into the left renal vein prior to DEV submucosal erosion
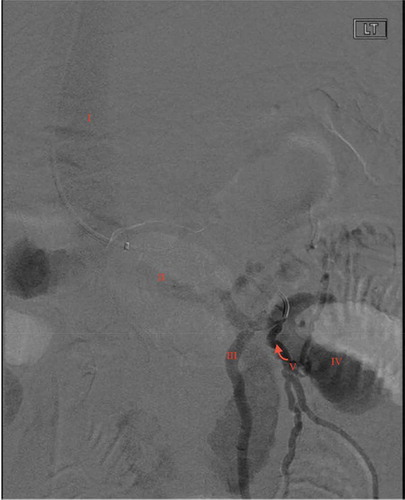
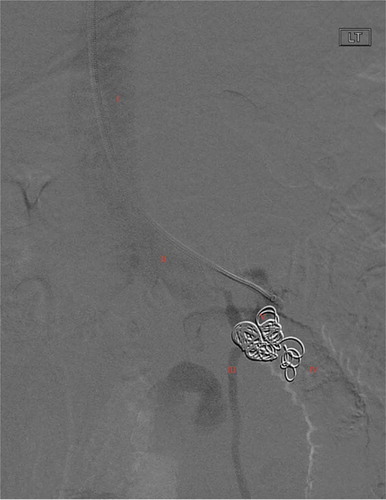
Figure 2. Venogram of distal duodenal ectopic varix status post varix outflow embolization with 0.35 interlock coil on HD3. Annotations as follows: I: IVC, II: Left renal vein, III: left gonadal vein, IV: absence of contrast into duodenum, V: 0.35 interlock coil filling DEV and outflow, absence of contrast distally in afferent feeders of DEV
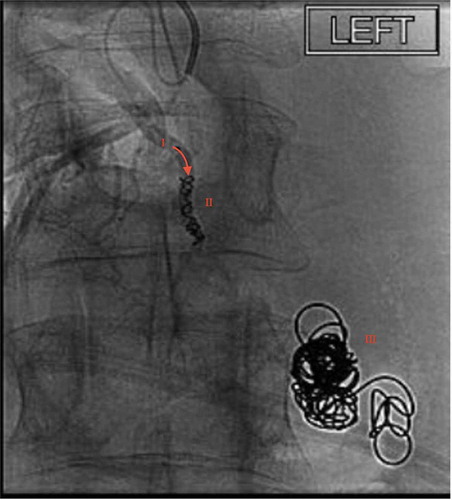
On HD 4 the patient underwent a TIPS procedure to decrease the risk of future hemorrhage. Once the TIPS procedure was placed there was a reduction in portal vein pressure from 22 mmHg to12 mmHg (reference normal; 5–10 mmHg) and a decrease in portosystemic gradient pressure from 10 mmHg to 6 mmHg (portal hypertension present if ≥ 6 mmHg, clinically significant if ≥10 mmHg) [Citation6]. A superior mesenteric venogram was performed which failed to demonstrate the varix. However, after selecting a medial branch of the superior mesenteric vein, a direction microcatheter and fathom microwire were used to select the branch feeding the varix. Venography was then performed which demonstrated a medial branch of the superior mesenteric vein feeding the large duodenal left renal vein varix with active extravasation into the duodenum. The varix was then thrombosed with Gelfoam slurry and a microcil was deployed into the duodenal varix inflow. A repeat angiogram was performed of the superior mesenteric vein confirming thrombosis of the treated vessel (). Following the embolization, there was no blood flow through the varix.
Figure 3. Fluoroscopy demonstrating successful microcoil placement in a medial branch of the superior mesenteric vein feeding the ectopic varix. Annotations as follows: I: medial branch of superior mesenteric vein, II: microcoil placement obstructing DEV afferent vein, III: previously deployed 0.35 interlock coil
Following the second embolization, the patient stabilized. On HD5 the patient returned to the operating room for ileocolonic anastomosis, fascial closure and placement of a wound vac. In total, during his hospitalization, he received over 80 units of blood products. He was discharged to home on HD 20.
3. Discussion
Ectopic varices are a diverse group of venous shunts located in the mesenteric vascular bed and exclude esophageal and gastric varices. Ectopic varices are an uncommon cause of GIB, representing only 2–5% of GIB, however the mortality in previous studies has been as high as 40% [Citation2]. The etiology for ectopic varices falls into two large categories: Type A and B. Type A ectopic varices are due to portal hypertension and Type B are from splanchnic venous occlusion. These occlusions can be due to thrombosis of the splenic vein, portal vein or mesenteric vein [Citation7]. In our case, the patient had both generalized portal hypertension and an occluded splenic vein.
Duodenal ectopic varices (DEV) are a subgroup of ectopic varices that includes a diverse set of vascular connections including portosystemic shunts, portal-portal anastomoses as well as mesenteric-to-portal shunts. The afferent feeders of DEV include the superior and inferior pancreaticoduodenal veins, cystic branches of the superior mesenteric veins, the gastroduodenal vein and the pyloric vein [Citation7–10]. There are numerous vascular sources that can act as the efferent branch of the DEV including the right gonadal vein, the capsular renal veins [Citation7,Citation8,Citation11], the left gonadal vein, directly into the inferior vena cava and the right renal vein [Citation7]. In our case, the duodenal varix outflow was into the left renal vein proper.
Although duodenal ectopic varices are most commonly located in the first and second parts of the duodenum, they can rarely be found in the more distal third and fourth parts of the duodenum as well. Duodenal ectopic varices represent 17% of the ectopic varices but are responsible for 25–33% of ectopic variceal bleeds [Citation7]. In our case, the patient had a distal DEV, with varix efferent flow into the left renal vein, and afferent flow from branches of the superior mesenteric vein in the setting of both elevated portosystemic gradient pressure and splenic vein obstruction.
The diagnosis of duodenal ectopic varices can be challenging, and physicians must have a high index of suspicion to make the diagnosis. The diagnosis of DEV is done typically by endoscopic examination; although duodenal ectopic varices can be difficult to visualize endoscopically due to their serosal and submucosal location [Citation8,Citation12,Citation13]. Further, standard upper endoscopy does not typically visualize the more distal portions of the duodenum where the ectopic varix was located in this case. In one of the larger case series to date, which included ten patients with duodenal ectopic varices, five of the patients presented with upper GI bleed whereas the other five patients had DEV incidentally detected. Nine out of the ten patients were diagnosed with DEV via endoscopy, but one was diagnosed via endoscopic ultrasound, which is not commonly used to diagnose the cause of GIB. All ten patients had DEV located in either the first or second portions of the duodenum, and only two of the ten patients had no history of esophageal varices [Citation14]. Our case presented with DEV in the distal duodenum and had no evidence of esophageal or gastric varices on endoscopy. Previous cases have reported DEV in the distal duodenum [Citation15–17], but they are extremely uncommon. Push enteroscopy has been used in previous case reports to detect ectopic varices [Citation18]. In our case push, enteroscopy did not visualize the varix. Other modalities used successfully to diagnose DEV include CT angiography [Citation19] and multislice helical CT scans with multiplanar reconstruction [Citation20,Citation21]. Diagnosis in our patient was made on the second interventional guided mesenteric angiogram after an upper endoscopy, two colonoscopies, two normal CT angiograms, and push enteroscopy did not elucidate the etiology of the bleeding.
Management of ectopic varices is extremely challenging. In particular, DEV represents a diverse group of variceal complexes and has highly variable underlying vascular anatomy [Citation22]. As such, no universal recommendation on treatment can be made. The management depends on clinical presentation, hemorrhage/variceal location, physician experience and etiology of portal hypertension [Citation23]. Treatment options for DEV include endoscopy guided management, surgical resection, transvenous obliteration, decompression utilizing TIPS, and decompression by recanalization of the occluded splanchnic vein [Citation7]. Case reports of DEV demonstrate effective treatment with TIPS alone or TIPS combined with varix embolization [Citation24,Citation25]. However, ectopic varices have been demonstrated to hemorrhage at lower portosystemic gradient pressure than esophageal or gastric varices [Citation26]. TIPS procedure alone may not be sufficient to eliminate the risk of future DEV bleeding. In a previous case series of five patients with bleeding DEV, three of the five patients presented with bleeding DEV despite the previous TIPS procedure to lower portosystemic gradient pressure. All patients in this case series were treated with TIPS and transvenous obliteration of the DEV [Citation27]. Rebleed rates after TIPS alone in the management of DEV are 21–37% and after transvenous obliteration is 13% [Citation7]. Our patient underwent TIPS and transvenous obliteration with coiling and gelfoam on consecutive days.
Our case illustrates several significant points. When a patient presents with bright red blood per rectum, no hematemesis and normal upper and lower endoscopies, consideration for hemorrhage of ectopic varix should be included in the differential. Successful management requires a multidisciplinary approach including surgeons, interventional radiologists, intensivists and gastroenterologists. Importantly, this report contributes to the small number of case reports of distal duodenal ectopic variceal hemorrhage. Further, our patient’s presentation of DEV with no known cirrhosis and without concurrent esophageal or gastric varices is uncommon.
Disclosure of Interest
The authors report no conflict of interest.
Geolocation
Boise, ID. USA
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Oakland K. Changing epidemiology and etiology of upper and lower gastrointestinal bleeding. Best Pract Res Clin Gastroenterol. 2019;42–43. 10.1016/j.bpg.2019.04.003
- Norton ID, Andrews JC, Kamath PS. Management of ectopic varices. Hepatology. 1998;2013(4):1154–1158. (was 3).
- Sharma M, Rameshbabu CS. Collateral pathways in portal hypertension. J Clin Exp Hepatol. 2012;2(4):338–352. (was 2).
- Kochar N, Tripathi D, McAvoy NC. Bleeding ectopic varices in cirrhosis: the role of transjugular intrahepatic portosystemic stent shunts. Aliment Pharmacol Ther. 2008;28(3):294–303.
- Khouqeer F, Morrow C, Jordan P. Duodenal varices as a cause of massive upper gastrointestinal bleeding. Surgery. 1987;102(3):548–552.
- Suk KT. Hepatic venous pressure gradient: clinical use in chronic liver disease. Clin Mol Hepatol. 2014;20(1):6–14.
- Saad WE, Lippert A, Saad NE. Ectopic varices: anatomical classification, hemodynamic classification, and hemodynamic-based management. Tech Vasc Interv Radiol. 2013;16(2):158–175.
- Hashizume M, Tanoue K, Ohta M. Vascular anatomy of duodenal varices: angiographic and histopathological assessments. Am J Gastroenterol. 1993;88(11):1942–1945.
- Tominaga K, Montani A, Kuga T. Combined balloon-occluded embolization for treatment of concurrent duodenal, gastric and esophageal varices: a case report. Gastrointest Endosc. 2001;53(6):665–668.
- Itzcham Y. Glickman MG: duodenal varices in extrahepatic portal obstruction. Radiology. 1977;124(3):619–624.
- Zamora CA, Sugimoto K, Tsurusaki M. Endovascular obliteration of bleeding duodenal varices in patients with liver cirrhosis. Eur Radiol. 2006;16(1):73–79.
- Sato T. Treatment of ectopic varices with portal hypertension. World J Hepatol. 2015;7(12):1601–1605.
- Sukigara M, Koyama I, Komazaki T. Bleeding varices located in the second portion of the duodenum. Jpn J Surg. 1987;17(2):130–135.
- Rana SS, Bhasin DK, Sharma V. Clinical, endoscopic and endoscopic ultrasound features of duodenal varices: a report of 10 cases. Endosc Ultrasound. 2014;3(1):54–57.
- Kunisaki T, Someya N, Shimokava Y. Varices in the distal duodenum seen with a fiberduodenoscope. Endoscopy. 1973;5(02):101–104.
- Khor V, Soon Y, Aung L. A case report of bleeding from a duodenal varix: rare cause of upper gastrointestinal bleeding. Int J Surg Case Rep. 2018;49:205–208.
- Anand R, Ali SE, Raissi D. Duodenal variceal bleeding with large spontaneous portosystemic shunt treated with transjugular intrahepatic portosystemic shunt and embolization: a case report. World J Radiol. 2019;11(8):110–115.
- Watson GA, Abu-Shanab A, O’Donohoe RL. Enteroscopic management of ectopic varices in a patient with liver cirrhosis and portal hypertension. Case Reports Hepatol. 2016;2016:2018642.
- Ibukuro K, Tsukiyama T, Mori K. Veins of retzius at CT during arterial portography: anatomy and clinical importance. Radiology. 1998;209(3):793–800.
- Yoshida S, Watabe H, Akahane M. Usefulness of multi-detector helical CT with multiplanar reconstruction for depicting the duodenal varices with multiple collateral shunt vessels. Hepatol Int. 2010;4(4):775–778. Published 2010 Jul 24.
- Weishaupt D, Pfammatter T, Hilfiker PR. Detecting bleeding duodenal varices with multislice helical CT. AJR Am J Roentgenol. 2002;178(2):399–401.
- Henry ZH, Caldwell SH. Management of bleeding ectopic varices. Tech Gastrointest Endosc. 2017;19(2):101–107.
- Akhter NM, Haskal ZJ. Diagnosis and management of ectopic varices. Gastrointestinal Intervention. 2012;1(1):3–10.
- Illuminati G, Smail A, Azoulay D. Bismuth H: association of transjugular intrahepatic portosystemic shunt with embolization in the treatment of bleeding duodenal varix refractory to sclerotherapy. Dig Surg. 2000;17(4):398–400.
- Almeida JR, Trevisan L, Guerrazzi F. Bleeding duodenal varices successfully treated with TIPS. Dig Dis Sci. 2006;51(10):1738–1741.
- Vangeli M, Patch D, Terrini N. Bleeding ectopic varices: treatment with transjugular intrahepatic porto-systemic shunts (TIPS) and embolization. J Hepatol. 2004;41(4):560–566.
- Saad WE, Lippert A, Schwaner S. Management of bleeding duodenal varices with combined TIPS decompression and trans-TIPS transvenous obliteration utilizing 3% sodium tetradecyl sulfate foam sclerosis. J Clin Imaging Sci. 2014;4:67. Published 2014 Nov 29.
