ABSTRACT
A Killian–Jamieson diverticulum is a ‘false’ diverticulum on the lateral side of the proximal cervical esophagus. They are much rarer than Zenker diverticula and can be difficult to diagnose. They are best visualized using fluoroscopy studies, however, the workup for patients admitted with dysphagia can be sometimes extensive and unnecessary, leading to costly hospital stays, longer admissions and exposure to excessive radiation. Herein, we present a patient previously diagnosed with a Killian Jamieson diverticulum, who presented with worsening dysphagia, odynophagia and neck swelling, and was found to have an unusual inferior extension of the diverticulum. This paper will recognize the role of fluoroscopy in diagnosing diverticula and identifying causes of dysphagia, and to also recognize the use of American College of Radiology ‘ACR’ Appropriateness Criteria to minimize unnecessary studies.
1. Introduction
A Killian–Jamieson diverticulum (‘KJD’) is a ‘false’ diverticulum on the lateral side of the proximal cervical esophagus [Citation1]. Although 25% of KJD are bilateral, they are typically unilateral [Citation2]. The true prevalence is unknown, but it seems that KJD is four times less common than Zenker’s Diverticula [Citation3]. It has been hypothesized that the KJD is a result of a functional outflow obstruction in the esophagus, causing an inappropriate constriction of proximal muscle fibers during swallowing [Citation1].
In the proximal esophagus, the KJD develops in an anterolateral muscular gap inferior to the cricopharyngeal muscle, called the KJ space [Citation1]. There is a muscular gap in the posterior portion of the cricopharyngeus, known as the Killian dehiscence, where Zenker’s diverticulum develop [Citation4]. These diverticula are anatomically distinct, although they both occur at sites of anatomic weakness [Citation4]. In KJD patients, there is no overflow aspiration or aspiration pneumonia, Aspiration pneumonia is seen in ~12% of Zenker’s Diverticula, as well as reflux into the hypopharynx [Citation4]. However, in KJD patients the high luminal pressure keeps the cricopharyngeus muscle closed, and thus there is no overflow aspiration in these patients [Citation1].
Diverticula are difficult to appreciate on endoscopy and so Barium studies are the gold standard [Citation5]. It is easier to estimate the size of the sac and approximate location to the cervical esophagus on barium studies, and thus these two diverticula can be only differentiated this way [Citation4]. A Zenker’s diverticulum is visualized in the posterior wall of the pharynx on lateral imaging and often with a prominent cricopharyngeal bar [Citation1]. The KJD is best seen on anteroposterior views below the cricopharyngeal muscle as a lateral outpouching [Citation1]. If the diverticulum is long and inferiorly displaced, it can often be difficult for a radiologist to distinguish between the two. Zenker’s diverticulum is known to be almost twice as big as KJD and typically presents with more severe symptoms [Citation5]. There are limited studies to assess the management and complications of KJD [Citation6]. However, some reports of unilateral KJD have been treated with esophagomyotomy [Citation1,Citation7].
2. Case
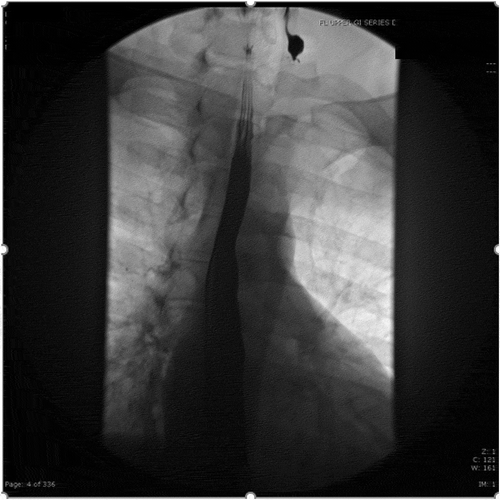
A 27-year-old male with Gastroesophageal reflux disease, obesity, gynecomastia, KJD () and nasal turbinectomy presented with left sided neck pain, new dysphagia to solids, odynophagia, and globus sensation. He was seen previously for similar episodes, for which he was given steroids and antibiotics for presumed esophagitis. He denied constitutional symptoms. His social and family histories were non-contributory. His vital signs and exam were unremarkable, but he had a mild leukocytosis, likely secondary to recent steroid use.
Figure 1. Fluoroscopy upper GI series with double contrast revealing new diagnosis of Killian Jamieson diverticulum in 2016
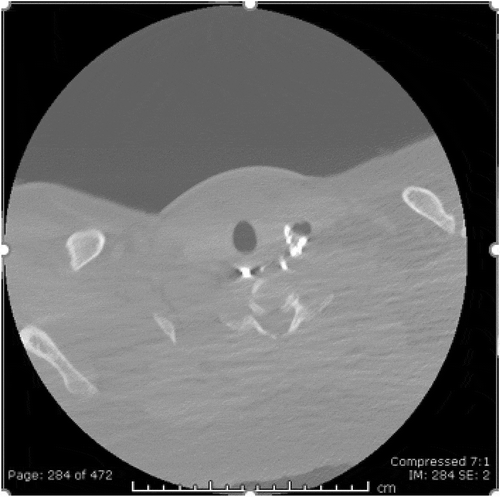
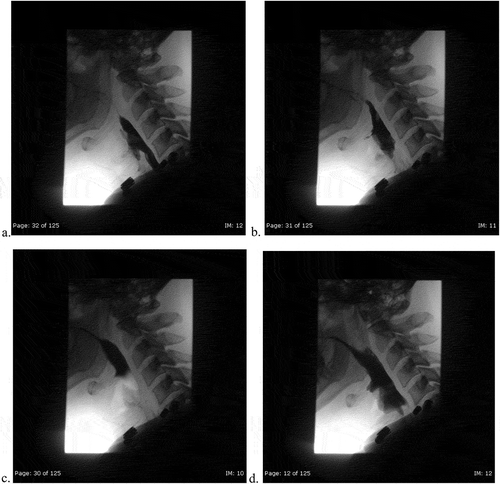
As he initially endorsed neck swelling and pain, he underwent an unremarkable Anterior-Posterior-Lateral X-ray to rule out food impaction/obstruction (). Bedside fiberoptic laryngoscopy was non-revealing. Due to his worsening dysphagia, odynophagia and history of KJD, Gastroenterology performed an esophagogastroduodenoscopy (EGD). The only significant finding was a diverticulum opening in the proximal esophagus and a distal ‘rent/tear.’ This was closed with hemo-clips. Following the EGD, the patient complained of acutely worsening odynophagia. He underwent a single contrast esophagram to rule out perforation and a diverticular leak ((ai)). Extraluminal contrast was found to the left of the diverticulum, with narrowed extension down to the hemo-clips. ((ai))A CT contrast of the neck illustrated extraluminal contrast at the level of C6/C7, ((ai))likely representing the esophageal diverticulum previously described(). At this time, a video-fluorographic swallow study was performed to better assess swallowing, aspiration and the role of diverticulum in the patient’s current dysphagia (). Speech pathology concluded that there was no aspiration and swallowing was normal. The patient was subsequently referred to ENT for further evaluation. He was determined to be a candidate for surgery and ultimately underwent esophagomyotomy. Although I absolutely agree that follow-up of this patient would help solidify that the diverticulum was in fact the cause of his symptoms, I was a medical student rotating through diagnostic radiology at the time. I followed the patient for his inpatient admission, even after I had left that particular rotation, however, after he was discharged by ENT there were no further internal records available. I kept in touch with a Radiologist there for updates, however, the patient had not returned for further imaging. One can assume that the patient has done well since the surgery, however, I don’t feel it would be fair nor honest of me to imply this in my paper, as I have no evidence to back this.
Figure 2. General diagnostic anterior-posterior and lateral views of the soft tissue neck. No radiographic evidence of food impaction. Slight deviation of trachea likely from rotation. Patent airway. Normal soft tissue structures. Mild ossification of stylohyoid ligament
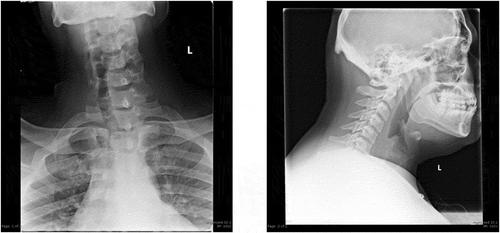
Figure 3a. (ad). Post-endoscopy single contrast esophagram. Anterior Views with attention to the neck initially with Gastrografin and then thin barium. 3D is a static image during phonation
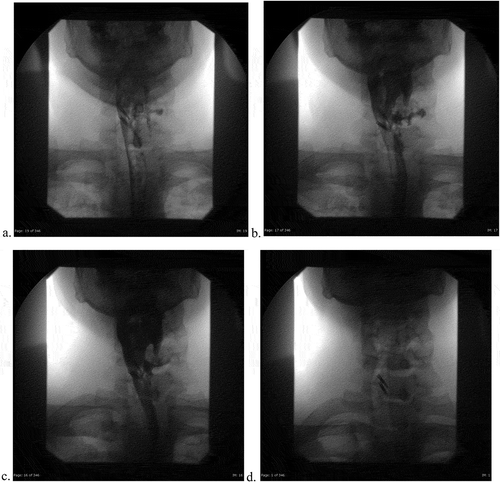
Figure 3b. (eh). Post-endoscopy single contrast esophagram. Lateral views using gastrografin, with attention to the vallecula and the diverticulum. Extrinsic impression and rightward deviation of the esophagus with extravasation of contrast around level of C6-C7. 3 H is a static image demonstrating normal morphology of the mucosa
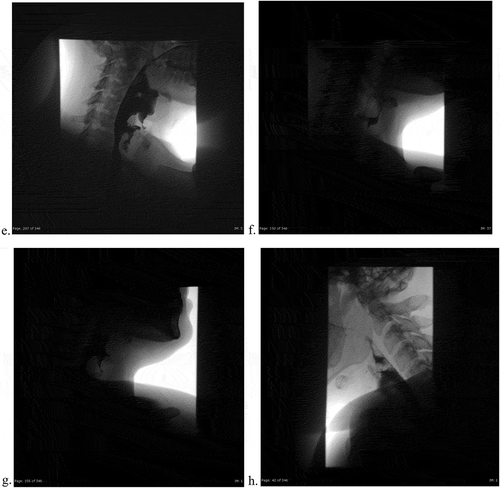
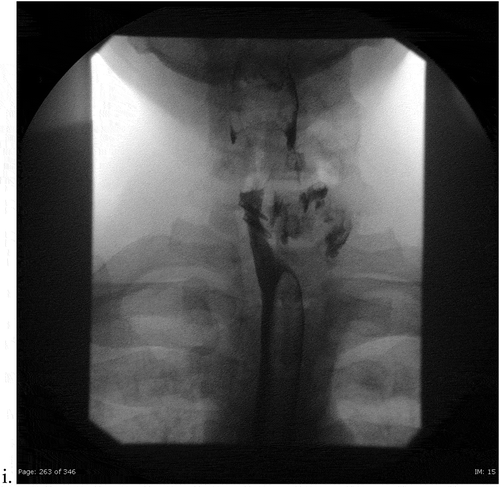
Figure 3c. (i). Post-endoscopy single contrast esophagram. Frontal view using thin barium, demonstrating extraluminal contrast with esophageal deviation to the right
3. Discussion
Fluoroscopy utilizes X-ray to produce real time images for diagnostic studies and/or to guide therapeutic procedures [Citation8]. Barium uses fluoroscopy to produce high radiation for extended periods of time and so careful history taking is warranted to prevent unnecessary exposure. Video-fluoroscopy (VF) or rapid sequence imaging can both be used. All of these tests can diagnose gastroesophageal reflux (GER) with Valsalva and leg raise techniques [Citation8]. The ACR Appropriateness Criteria provides different algorithms for variants of dysphagia. The first line test for those with dysphagia from an attributable cause, such as swallowing dysfunction or diverticulum, is a Modified Barium Swallow (MBS). An MBS uses VF to assess oropharyngeal swallowing function using various consistencies of barium [Citation8]. It is used in combination with static/dynamic images to evaluate structural abnormalities in the pharynx, such as pharyngeal tumors and diverticula [Citation8]. Speech therapy can also use these images to assess the efficacy of rehabilitation strategies. Single or biphasic esophagrams are second line choices, useful for evaluating oropharyngeal function and structure, respectively [Citation8]. A biphasic esophagram utilizes gas producing crystals to induce gastric/esophageal distention to better assess the mucosal anatomy. If a patient continuously drinks thin barium while prone, an esophagram is an excellent tool for detecting lower esophageal rings and strictures [Citation8]. Esophagram is also first line for retrosternal dysphagia and epigastric pains. Biphasic esophagrams are 96% sensitive for diagnosing esophageal and GEJ malignancies, implying that EGD is not warranted to rule out suspected tumors in those who had normal findings on radiological examinations [Citation8]. However, if multiple biopsies are taken during EGD, the sensitivity for detecting subclinical esophagitis, reflux and malignancies is higher than esophagram [Citation8]. Biphasic esophagrams are 95% sensitive for detecting peptic strictures and sometimes reveal strictures missed on EGD. EGD cannot determine if motility disorders are present. For post-operative patients, including patients status post EGD, a single contrast study might be warranted if there are concerns for fluid collections, anastomotic leaks, perforation and abscess [Citation8]. Esophagrams are highly specific, but not sensitive; however, use with CT contrast increases the sensitivity from 36% to 100% [Citation8]. Lastly, upper GI series are used to examine the esophagus, stomach and duodenum in patients with symptomatic GER, epigastric pain and dyspepsia. They can diagnose gastritis, duodenitis, peptic ulcer disease, hiatal hernias and varices [Citation6].
The challenge in this case was that the images during this hospital admission differed from his upper GI series in 2016. The diverticulum was narrower and more appeared to extend more inferior than in previous studies. Oddly, the extravasation of contrast into the soft tissue was on the opposite side of the clips, suggesting it was unlikely for there to be a perforation secondary to clip placement. Rather, there was a connection between the diverticulum and the amorphous pooling of contrast at level of the clips. It was difficult to determine which imaging modalities were most appropriate. Our patient could have been categorized under Variant 1, ‘dysphagia with an attributable cause,’ in which MBS is the first line modality [Citation8]; however, our patient presented with multiple ‘red flag symptoms,’ such as his age, odynophagia, weight loss, and anemia necessitating an EGD as the first line investigation [Citation3]. With worsening dysphagia following the procedure, he was subsequently categorized under Variant 5, ‘Early postoperative dysphagia [Citation8].’ First line imaging under Variant 5 is a single contrast esophagram with CT contrast of the neck to rule out perforation. Because those studies were equivocal, he underwent MBS (Variant 1) to ascertain if the presence of the diverticulum was causing his symptomology. Although the patient appropriately followed the ACR pathways, he was still exposed to high doses of radiation and underwent multiple studies.
4. Conclusion
In conclusion, it is important to have a protocol for the workup of dysphagia in hospitalized patients, because although dysphagia accounts for a small portion of hospital admission, it is associated with a longer hospital stay, increased cost of hospitalization and increased risk of mortality compared to other diagnoses [Citation3]. Additionally, the use of the ACR appropriateness criteria will help cut the cost and length of stay by aiding in a faster diagnosis and reducing the amount of radiation. Thus, the intent of this case report was to introduce a rare case that utilized ACR criteria variants appropriately and to acknowledge the role and benefits of various fluoroscopy methods.
Consent
As this is a case report, no consent was needed for the purpose of this paper.
Acknowledgments
I’d like to acknowledge the patient for allowing me to participate in the case and share our findings with our colleagues. I’d also like to thank the Radiology Department at Einstein Hospital for their support in writing and submitting this article.
Disclosure statement
The authors report no conflict of interest. Ethical review is not necessary, because this is a case report. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Boisvert RD, Bethune DC, Acton D, et al. Bilateral Killian-Jamieson diverticula: a case report and literature review. Can J Gastroenterol. 2010;24(3):173–174.
- Adams KM, Mahin KE. Killian-Jamieson diverticulum. J Am Osteopath Assoc. 2015;115(11):688.
- Altman KW. Dysphagia evaluation and care in the hospital setting: the need for protocolization. Otolaryngol Head Neck Surg. 2011;145(6):895–898.
- Rubesin SE, Levine MS. Killian-Jamieson diverticula: radiographic findings in 16 patients. AJR Am J Roentgenol. 2001;177(1):85–89.
- Grant PD, Morgan DE, Scholz FJ, et al. Pharyngeal dysphagia: what the radiologist needs to know. Curr Probl Diagn Radiol. 2009;38(1):17–32.
- Cook IJ, Kahrilas PJ. AGA technical review on management of oropharyngeal dysphagia. Gastroenterology. 1999;116(2):455–478.
- Kim DC, Hwang JJ, Lee WS, et al. Surgical treatment of killian-jamieson diverticulum. Korean J Thorac Cardiovasc Surg. 2012;45(4):272–274.
- Criteria ACoRA. Narrative evidence & rating table, ACR appropriateness criteria. Dysphagia. 2018.