ABSTRACT
Background: Endoscopic ultrasound guided celiac plexus neurolysis (EUS- CPN) has been reported to be an effective way to help with pain in pancreatic cancer patient. The aim of our updated meta-analysis is to assess the efficacy of pain relief in patients with pancreatic cancer who underwent EUS guided neurolysis.
Methods:
Pooled proportions were calculated using both Mantel-Haenszel method (fixed effects model) and DerSimonian Laird method (random effects model). The heterogeneity among studies was tested using Cochran’s Q test based upon inverse variance weights.
Results: Initial search identified 176 reference articles, of which 34 were selected and reviewed in detail. Sixteen studies that met the inclusion criteria were included in this analysis. The mean age of patients undergoing neurolysis was 56.31 ± 19.72 years. Number of males, N = 563 (57.4%), was higher than the number of females, N = 417 (42.5%). The pooled proportion of patients who showed pain relief with EUS-guided neurolysis was 71% (95% CI = 68–74). Bias calculated using Begg–Mazumdar was not significant (p = 0.8). In a subgroup analysis, when comparing the central and bilateral techniques, the pooled proportion of patients with pain relief was 66% (95% CI = 61–71) and 57% (95% CI = 48–67), respectively.
Conclusions: Our results show that EUS guided CPN could provide relief in as much as 70% of patients with central neurolysis technique having some edge over peripheral neurolysis. Further larger scale randomized controlled trials may further help to elaborate the efficacy of central vs peripheral neurolysis.
1. Introduction
Pancreatic cancer is usually diagnosed at an advanced stage and therefore holds one of the worst prognosis among solid organ malignancies. Severe abdominal and back pain is one of of the most common clinical manifestations of pancreatic cancer and the occurrence and severity of pain directly correlates with cancer progression [Citation1]. Non-steroidal anti-inflammatory drugs and opioids have routinely been used for pain management; however, side effects of opioid therapy such as sedation, constipation, respiratory depression, dependence and central hyperalgesia can significantly affect the quality of life. Refractoriness to opioid therapy leads to dose increments and dependence, thereby entering into a vicious cycle and further worsening of the opioid related side effects [Citation2].
First reported in 1914 as an intraoperative procedure, celiac plexus neurolysis has proved to be an alternative or adjunctive intervention for pain management. Traditionally, CPN has been performed under fluoroscopic, ultrasonographic or computed tomography imaging guidance [Citation3]. In 1996, a relatively safer, accurate and convenient technique was introduced by Faigal et al. and Wiersema and Wiersema, in the form of endoscopic ultrasonography guided celiac plexus neurolysis (EUS-CPN) [Citation4,Citation5]. Utilizing real-time imaging and Doppler assessment of intervening blood vessels provides the EUS-guided CPN an edge over other techniques [Citation3].
EUS-CPN usually uses a combination of a local anesthetic such as bupivacaine and a neurolytic agent in the form of absolute ethanol or phenol. It can be performed in two ways: central approach or bilateral approach. In the central technique, the neurolytic agent is injected at the base of the celiac artery; in the bilateral technique, the neurolytic agent is injected on both sides of the celiac artery [Citation6].
In this meta-analysis and systematic review, our objective is to determine the efficacy of pain relief in pancreatic cancer patients who underwent EUS-guided CPN. The outcomes were assessed using the latest studies performed in the last decade.
2. Methods and materials
2.1. Study selection criteria
Studies using EUS-guided CPN for pain control in patients with pancreatic cancer were selected.
The inclusion criteria were: (1) studies performed on pancreatic cancer patients after 2009; (2) studies utilizing EUS-guided intervention; (3) study designs including randomized clinical trials, non-randomized clinical trials, prospective studies and retrospective clinical studies. The exclusion criteria were as follows: (1) studies on patients with chronic pancreatitis; (2) other modalities of CPN including fluoroscopy and percutaneous (3) abstracts presented only in conferences; (4) studies on EUS-guided celiac plexus block.
2.2. Data collection and extraction
A thorough literature search was conducted in the Medline, PubMed, Ovid journals, Google Scholar, and Cochrane Central Register of Controlled Trials & Database of Systematic Reviews for the years 2009 to December 2020. The search terms used were endoscopic ultrasound, endoscopic ultrasonography, celiac plexus neurolysis and pancreatic cancer. Two authors independently searched and extracted the data into an abstraction form. Any difference was resolved by mutual agreement.
2.3. Statistical analysis
This meta-analysis was performed by calculating pooled proportions of pancreatic cancer patients who experienced pain relief with EUS-guided CPN. Pooled proportions were calculated using both the Mantel–Haenszel method (fixed effects model) and the Dersimonian Laird method (random effects model) [Citation7,Citation8].
In order to exhibit the point estimates in each study in relation to the summary pooled estimate, Forrest plots were drawn. In the Forrest plots, the width of the point estimates represents the weight assigned to that particular study. Heterogeneity between studies was evaluated using Cochran’s Q test based upon inverse variance weights, and heterogeneity was quantified using I2 statistic [Citation9].
Both Harbord-Egger bias indicator [Citation10] and Begg-Mazumadar bias indicator were utilized to test the publication and selection bias on the summary estimates [Citation11]. Publication bias was further evaluated by constructing funnel plots [Citation12,Citation13].
3. Results
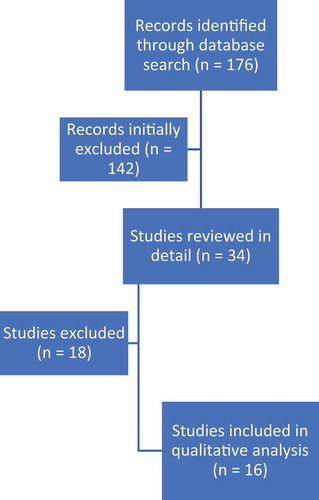
An initial search identified 176 articles, out of which 142 studies were initially excluded (studies that involved chronic pancreatitis patients and studies presented as abstracts in conferences). Thirty-four relevant studies were selected and reviewed in detail, of which 18 studies that utilized celiac plexus block technique were excluded and only 16 studies met the final inclusion criteria [Citation14–29]. shows the search results. The mean age of patients undergoing neurolysis was 56.31 ± 19.72 years. Number of males, N = 563 (57.4%), was higher than the number of females, N = 417 (42.6%). All the pooled estimates given are estimates calculated by the fixed effect model.
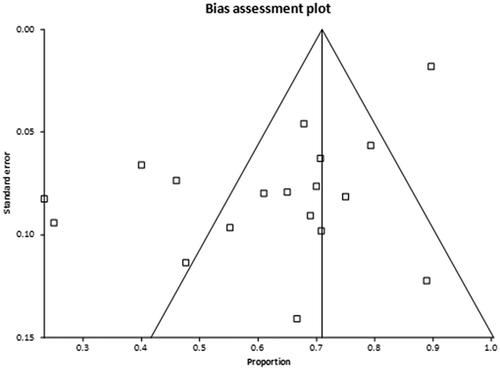
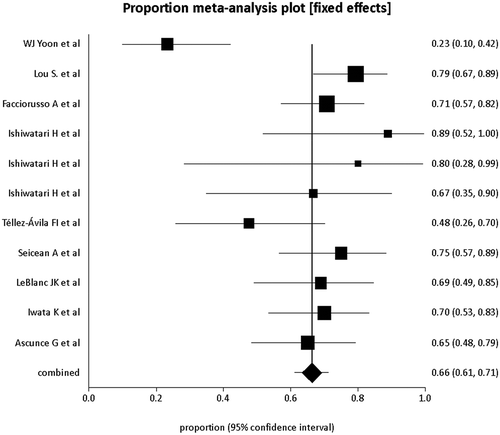
The pooled proportion of patients who showed pain relief with EUS-guided neurolysis was 71% (95% CI = 68–74). A Forrest plot showing the summary estimates is shown in . Publication bias calculated using the Harbord-Egger bias indicator gave a value of −4.36 (92.5% CI = −7.07 to −1.65, p = <0.05). The Begg-Mazumdar indicator gave a Kendall’s tau b value of −0.02 (p = 0.8), suggesting no publication bias. The funnel plot in shows no publication bias for EUS-guided CPN studies for pancreatic cancer pain.
Figure 2. Forrest plot showing the individual study proportion of pain relief in relation to pooled proportion of pain relief in pancreatic cancer patients
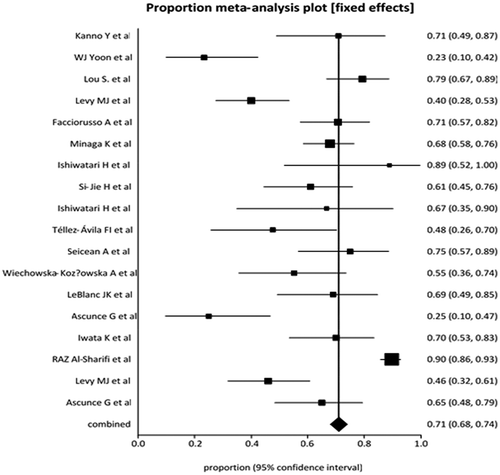
Figure 3. Funnel plot evaluating the effect of publication bias on EUS-guided CPN studies for pancreatic cancer pain
A subgroup analysis was also performed to further evaluate the proportion of pain relief with central injection compared to that with bilateral injection. With bilateral injection technique, the pooled proportion of patients with pain relief was 57% (95% CI = 48–67). On the other hand, with the central injection technique, the pooled proportion of patients with pain relief was higher at 66% (95% CI = 61–71). A comparison between forrest plots for both techniques is shown in .
Figure 4. Forrest plots showing the individual study proportion of pain relief in relation to pooled proportion of pain relief in pancreatic cancer patients undergoing EUS-guided CPN with central technique
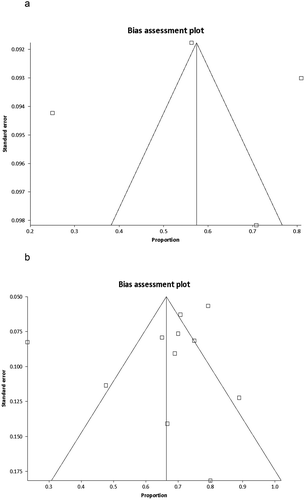
Publication bias calculated using the central technique using the Harbord-Egger bias indicator gave a value of −0.4 (92.5% CI = −4.39 to 3.59, p = 0.84). The Begg-Mazumdar indicator for bilateral and central technique gave Kendall’s tau b value of 0 (p = 0.75) and −0.16 (p = 0.44), respectively, suggesting no publication bias. Funnel plots in do not show any publication bias for either techniques.
4. Discussion
EUS CPN is markedly efficacious in the management of abdominal pain in pancreatic cancer patients and therefore should be considered at an early stage. It is imperative to understand the distinction between EUS-guided celiac plexus block (CPB) and celiac plexus neurolysis (CPN). CPB involves injecting an anesthetic with or without a steroid, whereas CPN involves injecting a neurolytic agent such as ethanol or phenol with or without an anesthetic. EUS CPN triggers an inflammatory process leading to fibrosis and therefore is recommended to avoid in cases like chronic pancreatitis, that may involve use of surgery eventually [Citation30]. All the studies presented in our meta-analysis used varying volumes of bupivacaine and either phenol or ethanol for EUS CPN.
Our study reflects that for pancreatic cancer patients, EUS-guided CPN gave pain relief in 70% of the patients, regardless of the technique. Moreover, for those treated with the central technique, it was 66% and for those treated with bilateral technique, 57%. A previous meta-analysis published in 2009 [Citation31] reported pain relief in 85% of the patients. A recently published meta-analysis on EUS-guided CPN in pancreatic cancer patients compared response rates at different time points post procedure and reported treatment response in 68% of the patients in week 2% and 53% of the patients at week 4. When comparing bilateral with central technique, it did not show a significant difference in treatment response at 2 weeks [Citation32].
For our meta-analysis retrospective studies, randomized and non-randomized clinical trials and prospective studies were included. Abstracts were not used during the analysis. Publication and selection biases are estimated by bias indicators and the use of funnel plots. In this meta-analysis, we used the Harbord-Egger bias indicator and the Begg-Mazumdar bias indicator [Citation10,Citation11] which showed no statistically significant bias. Furthermore, the point estimates were equally distributed on either side of the midline in our funnel plot, indicating no significant publication bias.
Some of the common adverse events associated with EUS CPN include transient diarrhea, hypotension, exacerbation of pain and inebriation. A review published in 2014 by Alvarez-Sanchez et al. reported complications in 21% cases of EUS CPN out of a total of 661 cases. In this review, major complications accounted for only 0.2% of the procedures [Citation33].
This meta-analysis and systematic review has several strengths that includes solid inclusion and exclusion criteria and a comprehensive search strategy. Every study included was of high quality and low publication bias. Our meta-analysis also included retrospective studies in addition to prospective studies and randomized/non-randomized clinical trials.
However, our meta-analysis is not without a few limitations. First, the definition of treatment response was not consistent between studies. Second, most studies did not differentiate between somatic pain and visceral pain. The importance of this lies in the fact that celiac plexus does not transmit somatic pain. Third, our meta-analysis did not measure pain relief at different time points post procedure. Finally, we excluded abstracts that lead to reduction in our patient population.
5. Conclusions
In conclusion, EUS-guided CPN is emerging as one of the mainstay therapies in pain management of pancreatic cancer patients refractory to opioid analgesics. It has its share of adverse events; however, the rate of complications is low and the benefits seem to outweigh the risk. Our results show that EUS-guided CPN could provide relief in as much as 70% of the patients with central neurolysis technique having some edge over peripheral neurolysis. The introduction of EUS-guided CPN early in the disease course of pancreatic cancer can prove to be more beneficial, and further research and clinical trials need to be conducted along this pathway.
Disclosure of potential conflicts of interest
No potential conflict of interest was reported by the author(s).
Acknowledgments
References
- Minaga K, Takenaka M, Kamata K, et al. Alleviating pancreatic cancer-associated pain using endoscopic ultrasound-guided neurolysis. Cancers (Basel). 2018;10(2):50.
- Urits I, Jones MR, Orhurhu V, et al. A comprehensive review of the celiac plexus block for the management of chronic abdominal pain. Curr Pain Headache Rep. 2020;24(8):42.
- Kothari TH, Kothari S, Kaul V. EUS-guided celiac plexus block and celiac plexus neurolysis. In: Adler MD, Facg AGAF, Fasge D, editors. Interventional endoscopic ultrasound. Cham: Springer; 2019;65-72. DOI:10.1007/978-3-319-97376-0_7
- Faigel DO, Veloso KM, Long WB, et al. Endosonography-guided celiac plexus injection for abdominal pain due to chronic pancreatitis. Am J Gastroenterol. 1996;91(8):1675.
- Wiersema MJ, Wiersema LM. Endosonography-guided celiac plexus neurolysis. Gastrointest Endosc. 1996;44(6):656–662.
- Yasuda I, Doi S, Mabuchi M. EUS-guided celiac plexus neurolysis. In: Mine T, Fujita R, editors. Advanced therapeutic endoscopy for pancreatico-biliary diseases. Tokyo: Springer; 2019;159-175. DOI:10.1007/978-4-431-56009-8_14
- Dorak MT 2007. Common concepts in statistics. Retrieved December, 16, 2007.
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clin Trials. 1986;7(3):177–188.
- Deeks JJ, Altman DG, Bradburn MJ, et al. Systematic reviews in health care: meta-analysis in context. In Statistical methods for examining heterogeneity and combining results from several studies in meta-analysis, 2nd. London: BMJ Publication Group; 2001;285-312.
- Harbord RM, Egger M, Sterne JA. A modified test for small‐study effects in meta‐analyses of controlled trials with binary endpoints. Stat Med. 2006;25(20):3443–3457.
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101.
- Sterne JA, Egger M, Smith GD. Investigating and dealing with publication and other biases in meta-analysis. Bmj. 2001;323(7304):101–105.
- Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54(10):1046–1055.
- Kanno Y, Koshita S, Masu K, et al. Efficacy of EUS-guided celiac plexus neurolysis compared with medication alone for unresectable pancreatic cancer in the oxycodone/ fentanyl era: a prospective randomized control study. Gastrointest Endosc. 2020;92(1):120–130.
- Yoon WJ, Oh Y, Yoo C, et al. EUS-guided versus percutaneous celiac neurolysis for the management of intractable pain due to unresectable pancreatic cancer: a randomized clinical trial. J Clin Med. 2020;9(6):1666.
- Lou S. Endoscopic ultrasound-guided celiac plexus neurolysis to alleviate intractable pain caused by advanced pancreatic cancer. Surg Laparosc Endosc Percutan Tech. 2019;29(6):472–475.
- Levy MJ, Gleeson FC, Topazian MD, et al. Combined celiac ganglia and plexus neurolysis shortens survival, without benefit, vs plexus neurolysis alone. Clin Gastroenterol Hepatol. 2019;17(4):728–738.e9.
- Facciorusso A, Di Maso M, Serviddio G, et al. Echoendoscopic ethanol ablation of tumor combined with celiac plexus neurolysis in patients with pancreatic adenocarcinoma. J Gastroenterol Hepatol. 2017;32(2):439–445.
- Minaga K, Kitano M, Sakamoto H, et al. Predictors of pain response in patients undergoing endoscopic ultrasound-guided neurolysis for abdominal pain caused by pancreatic cancer. Therap Adv Gastroenterol. 2016;9(4):483–494.
- Ishiwatari H, Hayashi T, Yoshida M, et al. EUS-guided celiac plexus neurolysis by using highly viscous phenol-glycerol as a neurolytic agent (with video). Gastrointest Endosc. 2015;81(2):479–483.
- Si-Jie H, Wei-Jia X, Yang D, et al. How to improve the efficacy of endoscopic ultrasound-guided celiac plexus neurolysis in pain management in patients with pancreatic cancer: analysis in a single center. Surg Laparosc Endosc Percutan Tech. 2014;24(1):31–35.
- Ishiwatari H, Hayashi T, Yoshida M, et al. Phenol-based endoscopic ultrasound-guided celiac plexus neurolysis for East Asian alcohol-intolerant upper gastrointestinal cancer patients: a pilot study. World J Gastroenterol. 2014;20(30):10512–10517.
- Téllez-Ávila FI, Romano-Munive AF, Herrera-Esquivel J, et al. Central is as effective as bilateral endoscopic ultrasound-guided celiac plexus neurolysis in patients with unresectable pancreatic cancer. Endosc Ultrasound. 2013;2(3):153–156.
- Seicean A, Cainap C, Gulei I, et al. Pain palliation by endoscopic ultrasound-guided celiac plexus neurolysis in patients with unresectable pancreatic cancer. J Gastrointestin Liver Dis. 2013;22(1):59–64.
- Wiechowska-Kozłowska A, Boer K, Wójcicki M, et al. The efficacy and safety of endoscopic ultrasound-guided celiac plexus neurolysis for treatment of pain in patients with pancreatic cancer. Gastroenterol Res Pract. 2012;2012:503098.
- LeBlanc JK, Al-Haddad M, McHenry L, et al. A prospective, randomized study of EUS-guided celiac plexus neurolysis for pancreatic cancer: one injection or two? Gastrointest Endosc. 2011;74(6):1300–1307.
- Ascunce G, Ribeiro A, Reis I, et al. EUS visualization and direct celiac ganglia neurolysis predicts better pain relief in patients with pancreatic malignancy (with video). Gastrointest Endosc. 2011;73(2):267–274.
- Iwata K, Yasuda I, Enya M, et al. Predictive factors for pain relief after endoscopic ultrasound-guided celiac plexus neurolysis. Digestive Endoscopy. 2011;23(2):140–145.
- Al-Sharifi RA. Role of endoscopic ultrasonography guided celiac plexus neurolysis in the management of pancreatic cancer pains. Iraqi Acad Sci J. 2009;8:1.
- Wyse JM, Battat R, Sun S, et al. Practice guidelines for endoscopic ultrasound-guided celiac plexus neurolysis. Endosc Ultrasound. 2017;6(6):369–375.
- Puli SR, Reddy JB, Bechtold ML, et al. EUS-guided celiac plexus neurolysis for pain due to chronic pancreatitis or pancreatic cancer pain: a meta-analysis and systematic review. Dig Dis Sci. 2009;54(11):2330–2337.
- Koulouris AI, Alexandre L, Hart AR, et al. Endoscopic ultrasound-guided celiac plexus neurolysis (EUS-CPN) technique and analgesic efficacy in patients with pancreatic cancer: a systematic review and meta-analysis. Pancreatology. 2021;S1424-3903(20)30876–0. DOI:10.1016/j.pan.2020.12.016
- Alvarez-Sánchez MV, Jenssen C, Faiss S, et al. Interventional endoscopic ultrasonography: an overview of safety and complications. Surg Endosc. 2014;28(3):712–734.