ABSTRACT
The clinical features of cardiac myxoma vary significantly from asymptomatic to severe cardiovascular complications like atrioventricular valve obstruction and thromboembolism depending on the location, size, and mobility of the tumor. Echocardiography is the diagnostic study of choice, and surgical resection is the method of choice to prevent complications.
We report a case of a 47-year-old female who presented with exertional dyspnea, malaise, and weight loss. Physical examination was significant for jugular venous distension, basal crackles in lungs, 2+ pedal edema, and rumbling diastolic murmur at apex. CT of the chest revealed a hypodense filling defect in the left atrium. Transthoracic echocardiogram showed a 5.5 × 4.5 cm mobile density, likely myxoma, attached to the interatrial septum and prolapsing into the left ventricle during the diastolic phase, causing functional mitral stenosis. She underwent a resection of cardiac myxoma. The histopathology report confirmed the diagnosis of myxoma, and post-operative recovery was uneventful.
1. Learning objective
Cardiac myxoma has a wide range of presentations which range from asymptomatic, constitutional symptoms, thromboembolism, intracardiac obstruction, and functional valvular stenosis. Echocardiography is a diagnostic study of choice and surgical resection is the method of choice to prevent complications. In cases presented with mitral stenosis, pulmonary hypertension, and right-sided heart failure, cardiac myxoma should be considered one of the differential diagnoses.
2. Introduction
Cardiac Myxoma (CM) is a primary cardiac tumor, which most commonly arises in the left atrium from a stalk connected to the fossa ovalis membrane in 75% of the patients, right atrium in 23%, and the ventricles in 2% of the patients. It varies in size between 1 and 15 cm in diameter [Citation1]. CM is mostly sporadic, but about 10% of cases are familial, and it is more common in females in the middle age with a female-to-male ratio of 2:1 [Citation2].
The clinical spectrum can be divided into obstructive complications, thromboembolic phenomenon, and constitutional symptoms dependent on the location, size, and mobility of CM. Large CM can cause functional atrioventricular valvular obstruction leading to congestive heart failure (CHF) and pulmonary hypertension (PH). CM originating from the ventricle can lead to left ventricular outflow tract obstruction resulting in syncope and sudden cardiac death. The thromboembolic phenomenon results from systemic embolization of the surface thrombi or tumor fragment and can result in stroke or ischemic complications. The constitutional symptoms are mainly due to the Interleukin-6 secreted by myxoma [Citation1,Citation3].
In our case, CM presented with severe obstructive complications as the tumor was prolapsing into the left ventricle, causing atrioventricular obstruction leading to mitral pseudostenosis resulting in severe PH, right-sided heart failure (RHF), and congestive hepatitis. Early recognition and prompt treatment of the uncommon presentation of CM is crucial to prevent fatal outcomes and hence, improve the quality of life.
3. Case presentation
A 47-year-old female presented with worsening exertional dyspnea, malaise, and unintentional weight loss of 10 Ibs for six weeks. The patient was able to perform regular activities with no prior physical limitations; however, at the time of presentation, climbing one flight of stairs resulted in severe shortness of breath. The patient denied any fever, chills, night sweating, cough, chest pain, dizziness, syncope, intermittent claudication, or amaurosis fugax. The patient reported smoking one pack per year for 25 years with no other past medical or cardiac illness. The clinical examination revealed a heart rate of 112 beats per minute, blood pressure of 121/84 mmHg, and temperature of 98.1°F. The patient was hypoxic with oxygen saturation of 84% on room air and required 2 L oxygen with a nasal cannula to maintain saturations above 92%. The rest of the physical examination was pertinent for regular S1, S2, apical diastolic rumbling murmur, raised jugular venous distension, bilateral basal crackles and wheezes in lungs, and bilateral 2+ pedal edema.
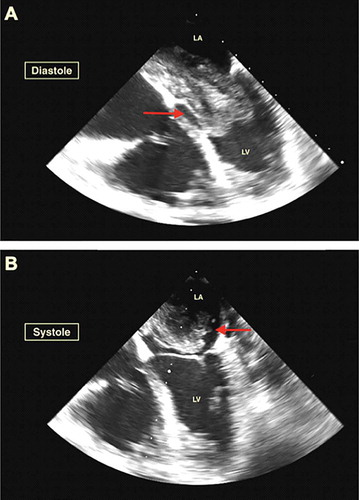
Laboratory workup showed brain natriuretic peptide of 266 pg/ml, alanine aminotransferase (ALT) of 1400 U/l, aspartate aminotransferase (AST) of 1660 U/l, and highest troponin-I level of 0.08 ng/ml, which further downtrend to 0.0 ng/ml. The electrocardiogram showed sinus rhythm without acute ST-segment or T wave abnormalities. The chest X-ray showed mild pulmonary congestion. CT with a contrast of the chest redemonstrated pulmonary congestion along with small bilateral pleural effusions and a giant hypodense filling defect in the left atrium, highly suspicious of myxoma (). Further investigation with transesophageal echocardiogram showed an ejection fraction of 55–60%, mild mitral regurgitation, severely increased right ventricle with decreased systolic function, severe tricuspid regurgitation, and severe PH with right ventricle systolic pressure of 61.2 mmHg. A giant 5.5 × 4.5 cm mobile density was attached to the interatrial septum with prolapse into the left ventricle during the diastolic phase leading to functional mitral valve pseudostenosis () (Supplemental online material 1).
Figure 1. Computerized Tomography of the chest with contrast showing a giant hypodense filling defect in the left atrium suspicious for a mass lesion
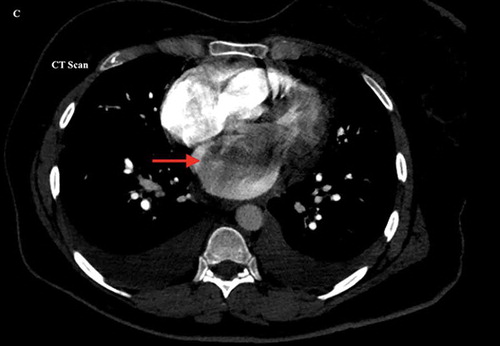
Figure 2. Panel A (Diastole) & B (Systole) Transesophageal echocardiogram revealed a giant 5.5 × 4.5 cm mobile density likely myxoma attached to the interatrial septum and prolapsing into the left ventricle during the diastolic phase causing functional mitral stenosis
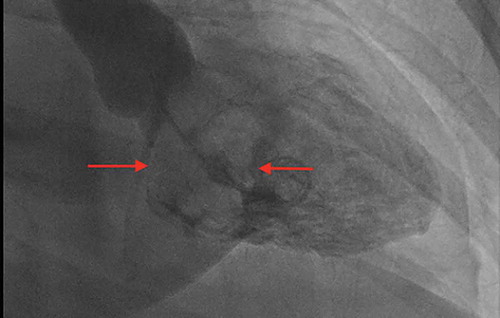
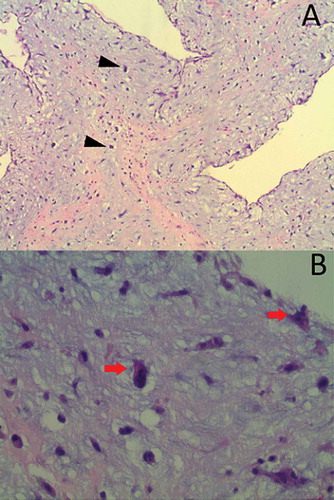
The patient was started on a beta-blocker to slow the heart rate and increase the mitral valve filling. The cardiothoracic surgery was consulted, and they recommended surgical removal of CM along with mitral and tricuspid valve repair. Preoperatively, the cardiac catheterization revealed normal coronary arteries, while the left ventriculogram redemonstrated the left atrial filling defect of the myxoma (). The patient underwent a resection of CM through a transseptal approach along with reconstruction of the septum with an autologous pericardial patch, mitral valve repair with a 28 mm annuloFlex posterior annuloplasty, and tricuspid valve repair with a 28 mm MC-3 ring. The resected mass was 7 cm in the greatest dimension, with a weight of 12 grams. The histopathology revealed ovoid to spindled and satellite cells arranged in cords, microtrabeculae, and nests within a prominent myxoid matrix and haemosiderin deposition, consistent with the diagnosis of myxoma ().
Figure 4. H&E staining at 100× (A) and 400× (B) revealed ovoid to spindled and satellite cells arranged in cords, microtrabeculae, and nests within a prominent myxoid matrix (arrow), and haemosiderin deposition (arrowheads) that was consistent with the diagnosis of myxoma
The postoperative recovery was uneventful, and the patient was discharged home in stable condition with no symptoms and had been advised to stop smoking permanently. Follow-up visits were scheduled at 1, 3, and 6 months to observe for recurrence.
4. Discussion
CM is a rare condition with an incidence of 0.5 per one million annually [Citation1]. Although it is a benign tumor, it can result in potentially life-threatening conditions. A detailed history and physical examination are crucial for early diagnosis. Cardiovascular symptoms, including chest pain, syncope, dyspnea, and angina, are the most common features in around 67% of the patients, out of which 28% can present with acute decompensated heart failure (ADHF) [Citation4]. Constitutional symptoms, including fever, malaise, arthralgia, and myalgia, are the second most common presentation in around 34%, while the embolic symptoms constitute up to 29% of the patients. Left-sided CM embolizes systemically to cerebral circulation (most common site, up to 30–40% of the patients), kidneys, and lower extremities, while the right-sided CM embolization occurs to pulmonary circulation [Citation5,Citation6]. In an asymptomatic patient, CM can be an incidental finding on imaging.
ADHF is one of the fatal complications resulting from the mechanical obstruction of the AV valve caused by CM, especially during the diastole. In our case, CM was prolapsing into the left ventricle during the diastole leading to mitral valve pseudostenosis resulting in severe PH and ADHF. Our patient had ALT and AST due to congestive hepatitis as a result of ADHF.
EKG findings may be non-specific in 20–40% of the patients [Citation7]. Left atrial hypertrophy is the most common findings on the EKG. Other findings may include non-specific ST-segment abnormalities and ventricular hypertrophy.
The echocardiogram is the diagnostic modality of choice to make the diagnosis; the presence of mobile mass with a stalk arising from the endocardial surface of the fossa ovalis is considered diagnostic without further imaging. In our patient, a giant 5.5 × 4.5 cm mobile density mas was attached to the interatrial septum with a thin stalk. The differentiation between CM and vascular tumors or thrombi can be done with contrast echocardiography perfusion or quantitative real-time perfusion imaging echocardiography [Citation8]. Cardiac magnetic resonance is helpful if CM cannot be visualized on echocardiography, and it is an important tool to identify the mass shape, density, and tissue signal intensity [Citation8]. Definitive diagnosis can usually be made after surgical resection using macroscopic and histopathology.
Surgical resection is the treatment of choice for myxomas with low morbidity and mortality. Long-term mortality (>30 days) was 11.5%, with no in-hospital mortality in a study done on 131 patients, and the mean survival was reported to be 130.6 ± 4.5 months [Citation9]. Our patient underwent a successful surgical resection along with valve repair. CM can recur in 2%-13% of the cases. Therefore, it is recommended to do a semi-annual follow-up for four years after the surgical resection with echocardiography [Citation8].
To our knowledge, only a few cases of CM were reported with severe obstructive complications due to mitral peusdostenosis resulting in severe PH and RHF. demonstrates age, sex, and imaging findings for similar cases of CM presented with mitral pseudostenosis. Our case emphasizes the importance of early diagnosis and proper treatment to reduce cardiovascular complications. This unusual presentation needs to be incorporated in the differential diagnosis of mitral pseudostenosis with secondary severe PH and RHF.
Table 1. Age, gender, and imaging features of patients with CM presented with mitral pseudostenosis (via multiple case reports)
Authors’ contributions
BA wrote the draft and final versions of the manuscript and edited it based on feedback. HK assisted in the modification of the drafts and the final version of the manuscript. HC and AM supervised the case and the write-up, making significant changes to the document, including the literature review. All authors read and approved the final manuscript.
Ethics approval and patient’s consent
Informed consent was obtained from the patient, and approval was obtained from McLaren Health Care privacy officer on 4/19/2021.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Bruce CJ. Cardiac tumours: diagnosis and management. Heart. 2011;97:151–160.
- Wen XY, Chen YM, Yu LL, et al. Neurological manifestations of atrial myxoma: a retrospective analysis. Oncol Lett. 2018;16:4635–4639.
- Theodoropoulos KC, Masoero G, Pagnano G, et al. Mitral pseudostenosis due to a large left atrial myxoma. J Geriatr Cardiol. 2018;15:244–245.
- Frizell AW, Higgins GL 3rd. Cardiac myxoma as a mimic: a diagnostic challenge. Am J Emerg Med. 2014;32:1399–1404.
- Pinede L, Duhaut P, Loire R. Clinical presentation of left atrial cardiac myxoma. A series of 112 consecutive cases. Medicine (Baltimore). 2001;80:159–172.
- Borcheni M, Kandah E, Abdelazeem B, et al. Unusual cause of stroke. Cureus. 2020;12:e11517.
- Burke A, Jeudy J Jr., Virmani R. Cardiac tumours: an update: cardiac tumours. Heart. 2008;94:117–123.
- Spartalis M, Tzatzaki E, Spartalis E, et al. Atrial myxoma mimicking mitral stenosis. Cardiol Res. 2017;8:128–130.
- Kuplay H, Kurc E, Mete EM, et al. Early and late results in surgical excision of primary cardiac tumors: our single-institution experience. 2018;26:177–182. Turk Gogus Kalp Damar Cerrahisi Derg. .
- Jagtap SV, Salunkhe P, Mane A, et al. Cardiac myxoma with cartilaginous differentiation-An uncommon variant presented as mitral stenosis. Indian J Pathol Microbiol. 2019;62:599–601.
- Strecker T, Agaimy A. Giant left atrial myxoma causing drop attacks by prolapsing into the mitral valve. Int J Clin Exp Pathol. 2012;5:996–999.
- Song J, Park S, Kim TY, et al. Large left atrial myxoma causing mitral annular dilation, functional mitral stenosis with concealed atrial septal defects. Korean J Anesthesiol. 2013;65:S67–69.
- Zuwasti U, Quarrie R, Allen E, et al. Severe functional mitral stenosis due to a left atrial myxoma masquerading as asthma. BMJ Case Rep. 2020;13. DOI:10.1136/bcr-2020-236876.