ABSTRACT
Tuberculous pericarditis is a rare extra-pulmonary manifestation of tuberculosis observed mainly in developed countries. It usually presents with concomitant tuberculous infection at a different site and, due to the lack of clinical specificity, diagnosis can be difficult. Thus, a diagnostic delay is frequent, entailing increased morbidity and mortality. The authors present a case of disseminated tuberculosis with predominantly cardiac symptoms with multiple negative samples for Mycobacterium tuberculosis, which evolved to constrictive pericarditis. With this case report, the authors emphasize the demand for a high index of suspicion for achieving a diagnosis and the importance of a multidisciplinary approach.
1. Introduction
Tuberculosis (TB) is a worldwide leading cause of morbidity and mortality. When TB infection affects two or more non-contiguous sites or presents in miliary form, it is called disseminated tuberculosis (DTB) [Citation1]. Tuberculous pericarditis (TBP) usually presents with vague and variable symptoms making it a diagnostic challenge. It is estimated that TB causes up to 4% of all acute pericarditis, with a mortality rate between 14 and 40% [Citation2]. An early diagnosis is crucial for improving the outcome of this disease.
2. Case report
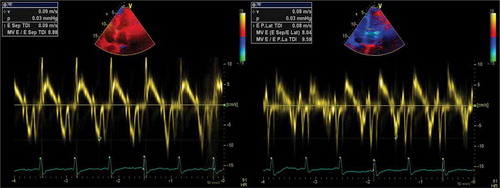
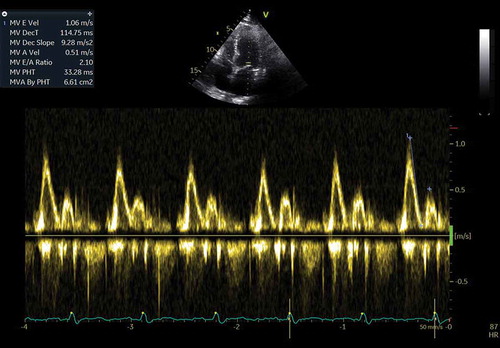
A 60-year-old white male presented for the third time within a month, at the emergency department (ED), with generalized oedema, orthopnoea, bendopnea and paroxysmal nocturnal dyspnoea. The patient had been medicated with diuretics but showed clinical deterioration. He had a past medical history of insulin-dependent diabetes, arterial hypertension and alcoholism. He worked as a taxi driver and denied recent travels, sexual risk behaviour or contact with apparently sick people. At admission, he was eupnoeic with a peripheral oxygen saturation of 98% and thoracic auscultation revealed bibasilar crackles. He had marked lower limb oedema and weighed 108 kg (14 kg above his usual weight). The initial analytical workup excluded anaemia and indicated normal levels of brain-type natriuretic peptide and non-elevated inflammatory parameters. The chest X-ray revealed an increased cardiac index and transthoracic echocardiogram showed moderate pericardial effusion and excluded compromised left ventricular function. In the first days of hospitalization, the patient performed several echocardiograms with signs of increased pericardial fluid, but without need of invasive procedures. Ten days later, the patient presented sudden clinical deterioration with cardiac tamponade (), for which pericardiocentesis was performed. Approximately 200 cc of hematic pericardial fluid were drained, an exudate according to Light criteria. Since the fluid was hematic, a cell count was not feasible and PCR for Mycobacterium tuberculosis (Mt) returned an inconclusive result. The smear was negative for acid-fast bacilli, Mt culture was negative and immunophenotyping excluded clonality. After two days, an additional pericardiocentesis was performed for signs of cardiac tamponade. The cardiac magnetic resonance revealed moderate pericardial effusion, with maximum thickness of 18 mm at the right ventricle, and a thickened pericardium (4 mm); it also revealed bilateral pleural effusion, focal thickening of the right pleura and delayed pericardial enhancement that spared the myocardium. Cardiothoracic surgery considered the pericardial window procedure inviable due to the thickness of the pericardium, presence of septated fibrin-rich effusion and of bilateral pleural effusion. The remaining etiological study showed: normal thyroid function (TSH 3.81 mU/L, T4L 1.17 ng/dL), an unremarkable autoimmune study and negative HIV serology. Diagnostic thoracentesis revealed a serohematic transudate (LDH 293 U/L, proteins 3.8 g/dL, ADA 32.8 UI/L, 2385 cells with 80% polymorphonuclear predominance) with negative acid-fast bacilli smear and Mt culture. A thoraco-abdominopelvic computed tomography scan showed a moderate bilateral pleural effusion, a thick pericardial effusion, scarce peritoneal effusion and diffuse oedema of the abdominal wall. Positron emission tomography scan () revealed a focus of moderate hypermetabolism in the posterior region of the right pleura and signs of discrete hypermetabolism at both the pleural and pericardial effusion. A thoracoscopy was performed and two pleural implants, with a yellowish hue and a reddish border, were observed. Both the pleural biopsy/pleural fluid revealed caseous granulomas (), although with a negative Mt PCR. Afterwards, despite a negative interferon gamma release assay (IGRA), and with Mt culture of pericardial and pleural effusion still pending (results were negative), a decision to start TB empirical treatment was made. To exclude concomitant pulmonary tuberculosis, three induced sputum samples were collected, one of which presented a positive Mt PCR. One week later the patient was discharged with a quadruple anti-tuberculosis regimen (HRZE) and oral prednisolone. One month after, the patient presented once again with decompensated heart failure (HF). Echocardiogram showed features suggestive of constrictive physiology ( and ) and right heart catheterization corroborated the constrictive pattern. Since then, the patient presented several times to the ED due to decompensated HF with worsening of functional capacity – NYHA IV, requiring hospitalization and adjustment of diuretic therapy. After one year, the patient underwent an antephrenic pericardiectomy. The postoperative period was complicated by acute kidney injury requiring renal replacement therapy and cardiogenic shock, ultimately resulting in patient death.
Figure 1. Transthoracic echocardiogram – subcostal window – showing a large circumferential pericardial effusion with right ventricular collapse
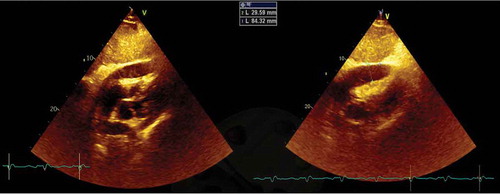
Figure 2. Positron emission tomography showing a focus of moderate hypermetabolism in the posterior region of the right pleura
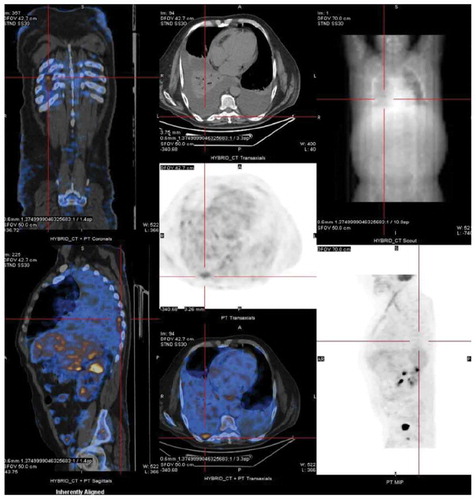
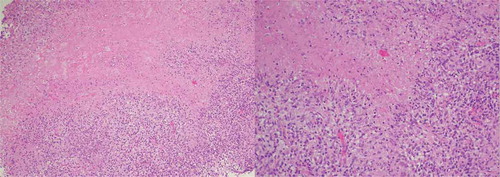
Figure 3. Pleural biopsy showing tuberculosis granuloma with a central caseous necrosis; magnification x100 and x200, respectively)
3. Discussion
Tuberculosis is a serious public health problem, responsible for high morbidity and mortality worldwide. Thus, its timely diagnosis and the early initiation of adequate treatment are fundamental to efficiently fight tuberculosis and avoid drug resistance.
Although conventional phenotypic methods of diagnosis and identification of Mycobacterium species are widely known and practiced, they require a long incubation period, can lead to inconclusive results and, in the case of extrapulmonary TB, may require invasive procedures for sample collection.
Tuberculous pericarditis is a type of extrapulmonary TB that usually manifests as one of three main clinical forms, namely pericardial effusion, constrictive pericarditis and a combination of both[Citation3]. The onset is most often insidious, but can be more acute and occasionally explosive with tamponade. In this case, an immunocompetent patient presented with HF, with none of the most frequently TB-associated complaints. Taking this into account, TB was not on top of the list of differential diagnosis. This hypothesis was strengthened over time, especially after pericardiocentesis showing bloody liquid. The diagnosis of TBP is usually based on four criteria, namely: (i) isolation of Mt from pericardial tissue or fluid cultures; (ii) demonstration of bacilli or granulomas in pericardial tissue on histopathological examination; (iii) granuloma in pericardial tissue in the presence of extracardiac tuberculosis focus; and (iv) response to specific treatment[Citation3]. Without the mentioned criteria, the diagnosis was only possible by the evidence of caseous granulomas on the pleural biopsy. Although culture is the gold standard for TB diagnosis, caseous necrosis has a high specificity for the disease, and its presence could justify the initiation of antituberculous therapy [Citation4,Citation5]. Diagnosis of extrapulmonary TB is considered a problematic process due to the paucibacillary nature of the specimens, lack of adequate clinical sample volumes and localization of disease in sites that are difficult to access [Citation6]. Even when the sample is optimal, culture is time-consuming (4–6 weeks) and has a variable sensitivity (50–100%) depending on time of collection and culture media type [Citation7].
In recent years, several alternatives to conventional methods have emerged. For example, genomic sequencing allows the detection of Mt DNA, as well as of the common mutations that confer drug resistance. Another type of assays – nucleic acid amplification (NAA) tests, are useful tools in identifying mycobacteria from clinical samples that tested positive for acid-fast bacillus with positive smear, but are less effective in paucibacillary specimens, which are frequently present in patients with extrapulmonary TB. As such, the use of these tests in extrapulmonary TB is controversial, and often their use will depend on local policies. In addition, the current data described in the literature regarding the use of Xpert in extrapulmonary TB, namely in TBP, is limited [Citation8]. Regardless of the performance of molecular tests, the phenotypic exams for culture isolation of mycobacteria are essential.
Matrix assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS) is a well-known technique that has been extrapolated to the analysis of Mycobacterium. This analysis allows the precise identification and discrimination of the genus and species of microorganisms, through comparison of their protein profile with a known reference spectral database. MALDI-TOF MS is simple, accurate, fast in terms of execution and delivery of results, and also a cost-effective technique when compared to other tuberculosis diagnostic methods [Citation9]. In combination with other conventional diagnostic methods, this technique can increase the sensitivity and accuracy of extrapulmonary TB diagnosis.
These advantages render it a promising tool, with the potential to be used in routine microbiology laboratories for the accurate classification of genus and species of mycobacteria, differentiation of non-tuberculous and tuberculous mycobacteria, enabling early treatment and thus preventing drug resistance.
According to the current literature and despite recent advances, this technique still needs to be improved, due to characteristics of the mycobacteria cell wall (hydrophobic nature, thick and waxy), as well as the low number of ribosomal proteins and the growth rate [Citation10]. Although protein spectra databases that are used to identify mycobacteria are developing rapidly, these are still limited and in need of expansion [Citation9].
Diagnostic tests based on clustered regularly interspaced short palindromic repeat (CRISPR) technology have also emerged as highly sensitive to identify Mt and diagnose pulmonary and extrapulmonary TB [Citation11]. Although this approach also yields information on the resistance profile to drugs, which is relevant for treatment choice, its application is a recent development that requires further validation.
Of note, none of the referred tests (genomic sequencing, NAA, mass spectrometry and CRISPR-based tests) are available at our laboratory.
In the case reported, following the evidence of pleural TB it was mandatory to exclude pulmonary TB. A positive PCR for Mt on an induced sputum sample confirmed the diagnosis of DTB (pericardial, pleural and pulmonary). Progression to constrictive pericarditis, even with optimal antituberculous therapy, is reported in up to 30% of cases[Citation12]. Rapid diagnosis and treatment of constrictive pericarditis are crucial to reduce mortality.
4. Conclusion
The authors alert to the atypical manifestations of DTB, where the multiple negative microbiologic tests can lead to a wide array of differential diagnosis and pose a diagnostic dilemma.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Wang JY, Hsueh PR, Wang SK, et al. Disseminated tuberculosis: a 10-year experience in a medical center. Medicine (Baltimore). 2007;86:39–46.
- Mayosi BM, Wiysonge CS, Ntsekhe M, et al. Mortality in patients treated for tuberculous pericarditis in sub-Saharan Africa. S Afr Med J. 2008;98:36–40.
- Reuter H, Burgess L, Van Vuuren W, et al. Diagnosing tuberculous pericarditis. QJM. 2006;99:827–839.
- Lee VY, Wong JT, Fan HC, et al. Tuberculous pericarditis presenting as massive haemorrhagic pericardial effusion. BMJ Case Rep. 2012;28. DOI:10.1136/bcr.03.2012.5967
- Ramírez-Lapausa M, Menéndez-Saldaña A, Noguerado-Asensio A. Tuberculosis extrapulmonar, una revisión [Extrapulmonary tuberculosis]. Rev Esp Sanid Penit. 2015;17:3–11.
- Mehta PK, Raj A, Singh N, et al. Diagnosis of extrapulmonary tuberculosis by PCR. FEMS Immunol Med Microbiol. 2012;66:20–36.
- Tyrrell FC, Budnick GE, Elliott T, et al. Probability of negative mycobacterium tuberculosis complex cultures based on time to detection of positive cultures: a multicenter evaluation of commercial-broth-based culture systems. J Clin Microbiol. 2012;50:3275–3282.
- World Health Organization meeting report of a technical expert consultation: non-inferiority analysis of Xpert MTB/RIF ultra compared to Xpert MTB/RIF. Geneva: World Health Organization; 2017.
- Neuschlova M, Vladarova M, Kompanikova J, et al. Identification of mycobacterium species by MALDI-TOF mass spectrometry. Adv Exp Med Biol. 2017;1021:37–42.
- Alcaide F, Amlerová J, Bou G, et al. How to: identify non-tuberculous Mycobacterium species using MALDI-TOF mass spectrometry. Clin Microbiol Infect. 2018;24:599–603.
- Ai JW, Zhou X, Xu T, et al. CRISPR-based rapid and ultra-sensitive diagnostic test for Mycobacterium tuberculosis. Emerg Microbes Infect. 2019;8:1361–1369.
- Chang SA. Tuberculous and infectious pericarditis. Cardiol Clin. 2017;35:615–622.