ABSTRACT
Hepatic encephalopathy (HE) is a reversible brain dysfunction caused by liver insufficiency and portosystemic shunting. Hepatic encephalopathy is a common complication of advanced liver disease and is on a rise with the increasing incidence of non-alcoholic steatohepatitis (NASH). Since partnership with nursing staff is a critical part of successful management of these complex patients, we conducted a survey assessing their knowledge regarding HE.169 nurses participated in the survey. We found that more than 30% of the nurses did not know that ammonia is one of the toxins responsible for causing hepatic encephalopathy. We also found that 20% of the nurses had difficulty answering questions regarding titration of lactulose to bowel movements. Dietary education is a significant area for improvement as 80% of the nurses wanted to restrict fat and carbohydrate intake in these patients. With this simple survey, we identified important knowledge gaps among experienced nurses at our institution. We believe that by improving knowledge through focused lectures, we can improve patient care and reduce the length of hospitalizations in patients with HE.
1. Introduction
Hepatic encephalopathy (HE) is defined as a reversible brain dysfunction caused by liver insufficiency and/or portosystemic shunting [Citation1]. Hepatic encephalopathy occurs as a complication of advanced liver disease or acute liver failure (ALF). In developed countries, the causes of advanced liver disease include excessive alcohol intake, non-alcoholic fatty liver disease (NAFLD), chronic viral hepatitis and autoimmune diseases [Citation2]. Drug-induced liver injury due to acetaminophen overdose accounts for the majority of the cases of ALF in the developed world. Other causes of ALF include acute viral hepatitis, ischemic liver injury from septic shock or circulatory failure [Citation3] With the world-wide rise in obesity and metabolic syndrome, non-alcoholic steatohepatitis (NASH) cirrhosis is expected to increase and as a result increase in the incidence of hepatic encephalopathy.
Development of HE is attributed to the accumulation of neurotoxic substances including ammonia in the bloodstream as well as the brain [Citation4]. Hepatic encephalopathy can be divided into covert HE and overt HE. Covert HE is the preclinical stage of overt HE and refers to cognitive dysfunction (such as difficulty in decision-making and psychomotor slowing) without disorientation [Citation5] while overt HE (OHE) is the presence of symptoms such as confusion, dysarthria, stupor, and coma. The development of OHE is a poor prognostic sign in the setting of chronic liver disease with the probability of survival of 44% at 1 year [Citation6]. In the setting of acute liver failure, the development of HE is a harbinger of progressive hepatic failure. In both acute and chronic settings, development of OHE is an indication for transplant evaluation.
HE is a cause of significant burden on our healthcare system. From 1993 to 2007, hospitalisation cost has increased from 13,000 USD to 30,000 USD/ hospital stay [Citation7] Between 2010 and 2014, US hospitalizations for HE increased by 24% with total inpatient costs over 11 USD billion [Citation8]. With the rising burden on the healthcare system, the hospital systems are invested in optimising the patient care. Given the chronic nature of this disease, a major focus now has been on improving self-care knowledge and compliance.
Bedside nursing staff spend the most concentrated time with the patient in the hospital setting which puts them in the unique position to recognise the early signs of OHE, alert the medical team as well as to educate the patients and their families on understanding and management of this condition. Increased awareness of the nursing staff of the subtle signs of OHE, would lead to lead to prompt recognition and appropriate response by the medical team to the patient’s changing clinical status in the inpatient setting. Similarly, education with regards to OHE provided by the nursing staff to the patients and their caregivers would lead to more effective outpatient management and decreased need for hospitalizations. Through this survey, we sought to determine baseline knowledge of inpatient nursing staff and identify areas for improvement in the management of patients with OHE.
2. Methods
The survey was conducted at Community Regional Medical Center (CRMC) in Fresno, California. CRMC is a 685-bed regional hospital and trauma center in Fresno, California. It hosts the medical education program of UCSF Fresno, part of a leading medical school in the United States. The survey was part of the quality improvement project aimed to identify and correct knowledge gaps of the nursing staff with regards to OHE with the goal of improving patient care, decreasing the length and the cost of hospitalizations. A survey was prepared using Qualtrics [Citation9]. It consisted of 5 multiple choice questions designed to explore the knowledge of the nursing staff on the appropriate blood tests in the setting of OHE, lactulose titration, nutritional requirements for patients with OHE and the timing of escalation of care. Additionally, the data regarding the participant’s hospital unit and years of experience were collected. The validity and reliability of the survey were assessed by the division of gastroenterology and hepatology as well as the department of nursing education. The survey was disseminated to the nursing staff via email. Survey participation was voluntary and anonymous. The responses were collected and stored in the Qualtrics software. The survey was disseminated to 350 nurses at CRMC.
3. Results
3.1. Demographics
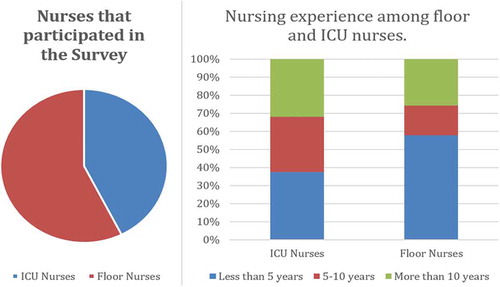
Out of 350 survey invitations, 169 nurses completed the full survey (). Seventy-two of the nurses worked in the intensive care unit (ICU) while 97 of the nurses worked in the hospital wards. Out of the 72 nurses in the ICU, 32% of the nurses reported more than 10 years of nursing experience while 38% of the nurses had been working for less than 5 years. Out of the ward nurses, about 58% of the nurses had less than 5 years of nursing experience, while 26% of the nurses had worked more than 10 years on the floors.
3.2. Survey Responses
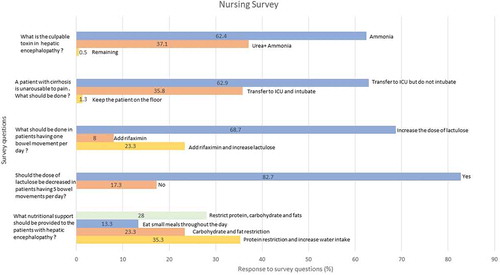
Which blood test is helpful to diagnose hepatic encephalopathy besides clinical features and physical examination?
69% of floor nursing staff vs 54% of ICU nursing staff chose ammonia, while 31% of floor and 44% ICU nursing staff chose to additionally check urea.
A patient admitted for management of hepatic encephalopathy progressively has been getting more confused. Yesterday the patient had incoherent speech and today he is found unarousable to pain. What will you do?
Most wanted to alert the attending for transfer to ICU (97.7% floor vs 100% ICU). 62.9% of the nursing staff wanted to hold the intubation vs 35.8% wanted the patient intubated emergently ().
A patient has been admitted for hepatic encephalopathy. He is still confused on day 2 of hospitalisation and has been having one bowel movement per day. What will you do?
71.1% of the nursing staff wanted to increase lactulose dose for less than one bowel movement daily, 21.9% wanted to add rifaximin and increase lactulose while 7% wanted to initiate rifaximin, without increasing lactulose.
A patient admitted to the hospital for hepatic encephalopathy is having 5 bowel movements per day while receiving lactulose. What would you do?
Most of the nursing staff (82.7%:82% ICU, 83% floor) wanted to decrease the frequency of lactulose while the remaining nurses wanted to continue the same dose of lactulose as before.
What is the best nutritional support for patients with cirrhosis/hepatic encephalopathy?
71.9% of nursing staff wanted to restrict protein, carbohydrate, or fat intake, while other nurses wanted to appropriately recommend frequent small meals with complex carbohydrates.
4. Discussion
Liver Cirrhosis is the ninth leading cause of death in the United states with 44,000 deaths alone in 2019 [Citation10]. Multi-disciplinary approach to care is of paramount importance in care of these patients. Regardless of the cause of cirrhosis, patients with hepatic encephalopathy have specific needs that should be addressed for a better outcome. Partnership with nursing staff is a critical part of successful management of these complex patients.
4.1. Toxins
- The pathophysiology of hepatic encephalopathy is multifactorial. The hypothesized neurotoxins include ammonia, tyramine, octopamine, manganese, GABA, etc., [Citation1]; however, ammonia is the most widely recognised toxin. Ammonia is produced by the bacteria in the gastrointestinal tract and is cleared by liver. In the setting of cirrhosis, combination of declining liver function and portosystemic shunting leads to decreased ammonia clearance [Citation11]. The ammonia crosses the blood brain leading to neuropsychiatric effects [Citation12]. Although most of the nursing staff in our survey elected ammonia, some additionally chose urea which is not one of the toxins associated with OHE. We believe that improving the knowledge of the nursing staff on the pathogenesis of OHE would help in effective management of these complex patients during the hospitalization and after discharge.
4.2. Medications for hepatic encephalopathy
- Lactulose and Rifaximin are the main medications used in management of hepatic encephalopathy. Lactulose reduces intestinal ammonia production and absorption. Lactulose leads to reduced intestinal pH which leads to conversion of ammonia produced by bacteria to ammonium ion which is unable to cross biological membranes and as a result leads to increased excretion of ammonia into the stool [Citation13]. The dose of lactulose is titrated to the number of the bowel movements with a goal of achieving 2–3 soft bowel movements every day [Citation14]. Adverse effects with more than 3 bowel movements include electrolyte abnormalities such as hypernatremia and hypokalaemia which can paradoxically worsen HE [Citation15,Citation16]. Given the need for titration of lactulose, partnership with nursing staff is critical to achieve optimal administration of this medication.
Rifaximin is an oral, non-absorbable antibiotic that achieves high concentration in the gut [Citation17]. It effectively eliminates the bacteria responsible for the production of ammonia leading to reduction in the amount of ammonia entering the bloodstream [Citation18]. A study by Bass et al [Citation19] indicated that combination of lactulose and rifaximin has been more effective in decreasing breakthrough OHE as well as hospital re-admissions as compared to lactulose alone. In our study, only 22% of the nurses wanted to add rifaximin and increase lactulose. We believe that raising awareness of the nursing staff on the effectiveness of combination therapy will be beneficial as they can educate the patients and their caregivers about the importance of each of these drugs leading to improved compliance and decrease in re-admissions for OHE
4.3. Nutritional Requirements
- Protein/calorie malnutrition is a common and underrecognized complication of cirrhosis [Citation20]. Besides the liver, muscle tissue also plays an important role in removal of circulating ammonia [Citation21]. Loss of skeletal mass may lead to decreased toxin clearance and as a result neuropsychiatric symptoms due to hepatic encephalopathy. The International Society for Hepatic Encephalopathy and Nitrogen Metabolism (SHEN) developed a consensus document in 2013. As per the document, dietary protein restriction should be avoided. There are studies which document that patients with hepatic encephalopathy can tolerate normoproteinemic diets and are able to benefit from them. Small frequent meals avoid undue gluconeogenesis in the liver and muscle. Amino acids are a substrate for gluconeogenesis and can lead to proteolysis of the skeletal muscle protein. This coupled with decreased protein synthesis is a frequent cause of sarcopenia in cirrhotics. As a result, small frequent meals with high protein were recommended by the society [Citation22].
Multiple studies have documented frailty in cirrhosis as an independent risk factor for worse outcomes such as increased days of hospitalisation and mortality while awaiting liver transplant [Citation23,Citation24]. Thus, special attention should be paid to the nutritional requirements of these patients especially protein. It has been recommended that the cirrhotic patients consume 35–40 Kcal/kg/day and 1.2–1.5 g/kg of protein/day [Citation25]. In our hospital, the nurses were not aware about these recommendations and most of the nursing staff wanted to restrict protein intake/carbohydrate. We believe this can be a considerable area of improvement and would improve clinical outcomes.
5. Conclusion
This is the largest survey that has been performed to identify inpatient nursing knowledge regarding management of HE. Although most nurses are able to recognize worsening symptoms of HE, many were not aware that ammonia is the culprit toxin. Nearly 70% of nurses wanted to up titrate lactulose without adding rifaximin in the cases of decreased bowel movements, which shows that knowledge regarding the combination therapy can be improved. As only 13% of nurses were aware that ‘small, high protein, frequent meals’ are recommended for HE patients, dietary education is a significant area for improvement. With this simple survey, we identified important knowledge gaps among experienced nurses at our institution. We believe that by improving knowledge through focused lectures, we can improve patient care and reduce length of hospitalizations in patients with HE.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Mandiga P, Foris LA, Bollu PC. Hepatic encephalopathy. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan [ Updated 2021 Mar 7]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK430869/
- Sharma A, Nagalli S. Chronic liver disease. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan [ Updated 2020 Jul 5]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK554597/
- Blackmore L, Bernal W. Acute liver failure. Clin Med (Lond). 2015;15(5):468–472.
- Ali R, Nagalli S. Hyperammonemia. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan [ Updated 2020 Aug 10]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK557504/
- Weissenborn K, Ennen JC, Schomerus H, et al. Neuropsychological characterization of hepatic encephalopathy. J Hepatol. 2001;34:768–773.
- Bohra A, Worland T, Hui S, et al. Prognostic significance of hepatic encephalopathy in patients with cirrhosis treated with current standards of care. World J Gastroenterol. 2020;26(18):2221–2231.
- Neff G. Pharmacoeconomics of hepatic encephalopathy. Pharmacotherapy. 2010 May;30(5 Pt 2):28S–32S. . PMID: 20412038.
- Hirode G, Vittinghoff E, Wong RJ. Increasing burden of hepatic encephalopathy among hospitalized adults: an analysis of the 2010-2014 national inpatient sample. Dig Dis Sci. 2019 Jun;64(6):1448–1457. Epub 2019 Mar 13. PMID: 30863953.
- Available from: www.qualtrics.com
- Available from: https://www.cdc.gov/nchs/fastats/liver-disease.htm
- Sawhney R, Jalan R. Liver: the gut is a key target of therapy in hepatic encephalopathy. Nat Rev Gastroenterol Hepatol. 2015 Jan;12(1):7–8. Epub 2014 Oct 28. PMID: 25348849.
- Ge PS, Runyon BA. Serum ammonia level for the evaluation of hepatic encephalopathy. JAMA. 2014;312(6):643–644.
- Prasad S, Dhiman RK, Duseja A, et al. Lactulose improves cognitive functions and health-related quality of life in patients with cirrhosis who have minimal hepatic encephalopathy. Hepatology. 2007 Mar;45(3):549–559. PMID: 17326150.
- Mukherjee S, John S. Lactulose. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan [ Updated 2020 Jul 10]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK536930/
- Hudson M, Schuchmann M. Long-term management of hepatic encephalopathy with lactulose and/or rifaximin: a review of the evidence. Eur J Gastroenterol Hepatol. 2019;31(4):434–450.
- Frederick RT. Current concepts in the pathophysiology and management of hepatic encephalopathy. Gastroenterol Hepatol. 2011;7(4):222–233.
- Scarpignato C, Pelosini I. Rifaximin, a poorly absorbed antibiotic: pharmacology and clinical potential. Chemotherapy. 2005;51(Suppl 1):36–66. PMID: 15855748.
- Flamm SL. Rifaximin treatment for reduction of risk of overt hepatic encephalopathy recurrence. Therap Adv Gastroenterol. 2011;4(3):199–206.
- Bass NM, Mullen KD, Sanyal A, et al. Rifaximin treatment in hepatic encephalopathy. N Engl J Med [Internet]. 2010 Mar 25;362(12):1071–1081.
- Periyalwar P, Dasarathy S. Malnutrition in cirrhosis: contribution and consequences of sarcopenia on metabolic and clinical responses. Clin Liver Dis. 2012;16(1):95–131.
- Jindal A, Jagdish RK. Sarcopenia: ammonia metabolism and hepatic encephalopathy. Clin Mol Hepatol. 2019;25(3):270–279.
- Amodio P, Bemeur C, Butterworth R, et al. The nutritional management of hepatic encephalopathy in patients with cirrhosis: international society for hepatic encephalopathy and nitrogen metabolism consensus. Hepatology. 2013;58:325–336.
- Lai JC, Rahimi RS, Verna EC, et al. Frailty associated with waitlist mortality independent of ascites and hepatic encephalopathy in a multicenter study. Gastroenterology. 2019 May;156(6):1675–1682. Epub 2019 Jan 19. PMID: 30668935; PMCID: PMC6475483.
- Sinclair M, Poltavskiy E, Dodge JL, et al. Frailty is independently associated with increased hospitalisation days in patients on the liver transplant waitlist. World J Gastroenterol. 2017 Feb 7;23(5):899–905. PMID: 28223735; PMCID: PMC5296207.
- Moss O. Nutrition priorities: diet recommendations in liver cirrhosis. Clin Liver Dis. 2019;14:146–148.