ABSTRACT
Dermatomyositis (DM) and polymyositis (PM) are both immune-mediated inflammatory myopathies known to occur in paraneoplastic syndromes associated with a new diagnosis of malignancy, most commonly breast, ovarian, lung, pancreatic, stomach, colorectal, and Non-Hodgkin’s lymphoma1 in DM and breast, lung, bladder cancer, and Non-Hodgkin’s lymphoma in PM. 2,3,4 While inflammatory markers such as creatine kinase (CK) may be elevated with either DM or PM, marked elevation is rare. Herein, we report a case of newly diagnosed pancreatic cancer presenting with inflammatory myopathy and marked CK elevation. We review the frequency of PM as a paraneoplastic syndrome, the association with marked CK elevation, and the association with pancreatic cancer.
1. Case report
A 66-year-old man with a history of diabetes mellitus type 2, hypertension, and hyperlipidemia, presented with severe, bilateral thigh pain. On further history, he reported 6 weeks of generalized fatigue, 4 weeks of progressively worsening weakness and loose stools in the setting of an 18-kg weight loss over the preceding 3 months. The patient had delayed seeking medical attention due to fear of contracting COVID-19 but ultimately presented after an acute worsening of his weakness, resulting in an inability to walk up the stairs in his home. His prior to admission medications included lisinopril, metformin, and atorvastatin, which he had been taking for several years. The patient was a retired teacher and lived with a roommate. He consumed 3–10 alcoholic beverages a week and had quit tobacco smoking 20 years prior. Previously, he had smoked one-half pack per day. His family history was notable for rheumatoid arthritis in his father.
On exam, the patient’s vital signs were normal and physical exams revealed a jaundiced appearance and three-fifths strength to hip flexion and extension bilaterally. The remainder of the exam was unremarkable. On initial laboratory assessment, white blood cell count was 11.9 k/mm3 (normal 4–10), total bilirubin 24.6 mg/dL (normal 0.1–1.2), direct bilirubin 13.0 mg/dL (normal 0.1–0.3 mg/dL), ALT 1053 U/L (normal <41), AST 2994 IU/L (normal 5–40), alkaline phosphatase 2893 IU/L (normal 40–129). Lipase, creatinine, and GFR were within normal limits. CK was elevated to 38,000 U/L (normal 39–308). The patient’s urine sample was tea-coloured on gross inspection. Urinalysis was performed although of limited utility as the pH, leukocyte esterase, nitrites, protein, glucose, ketone, bilirubin, and blood values were unable to be assessed due to interfering color. Regarding WBC and RBC counts, both returned at 0–2 (normal <2).
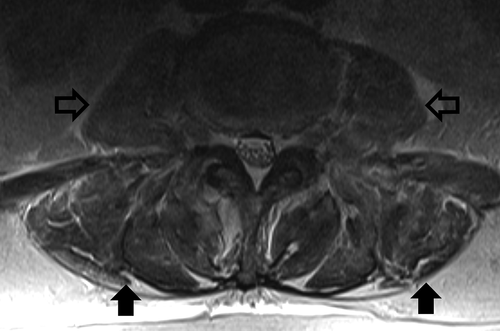
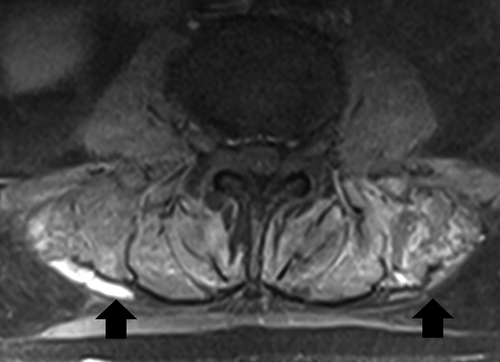
Given the elevation in CK, the patient’s statin was held, and he was started on aggressive fluid resuscitation. An abdominal CT scan showed a 3 cm obstructing pancreatic head mass with diffuse pancreatic, biliary, and gallbladder dilatation. Carbohydrate 19–9 antigens returned elevated at 8,782 U/mL (reference range 0–37 u/mL). Duodenal biopsy confirmed pancreatic adenocarcinoma. Given his significant bilateral lower extremity weakness, MRI lumbar spine was done to rule out metastasis. MRI revealed diffuse edema within the paraspinal and bilateral psoas musculature with associated patchy enhancement that was thought to represent sequela of myositis. No metastatic spinal cord compression was appreciated.
Despite early initiation of aggressive fluid resuscitation, the patient’s CK continued to rise. Given concern for inflammatory myositis, on hospital day 5 the patient was initiated on prednisone 80 mg/day. On hospital day 6, following initiation of steroids, his CK value plateaued at 75,000 U/L and began declining. Creatinine and GFR remained within normal limits despite elevated CK. On hospital day 12, the patient was seen by oncology and began chemotherapy with fluorouracil, oxaliplatin, and leucovorin in hopes of relieving his lower extremity weakness. He remained on prednisone therapy and his CK normalized on hospital day 14. His urine appearance improved from tea-coloured to clear yellow. The patient’s hip weakness was still present objectively, but subjectively he felt increased strength. He was discharged in stable condition to a skilled nursing facility and, after 3 weeks, was able to ambulate with a walker and returned home. He continued outpatient palliative chemotherapy with five total cycles of fluorouracil, oxaliplatin, and leucovorin and then one cycle of gemcitabine and abraxane. He died about 1 month later.
2. Discussion
We present a case of newly diagnosed pancreatic cancer presenting with inflammatory myopathy and marked elevation in CK. We review the frequency of PM as a paraneoplastic syndrome, the association with pancreatic cancer, and the association with marked elevation in CK.
2.1. Definitions
DM and PM are both immune-mediated inflammatory myopathies that typically present with symmetric, proximal muscle weakness. The primary differentiating factor between the two entities is the skin involvement associated with DM. Both processes can occur as paraneoplastic syndromes associated with a new diagnosis of malignancy [Citation1–3].
2.2. Epidemiology
The incidence of inflammatory myopathies ranges from 1.6 to 19 per million, with an estimated prevalence of 14 per 100,000[Citation4]. Women are more frequently affected than men[Citation5]. The average age of those affected by PM and DM is 50–60 years[Citation5]. The observed association between inflammatory myopathies and malignancy is more common in DM than PM and varies from 3% to 60%[Citation6].
The association of DM as a paraneoplastic syndrome has been well reported. Hill et al. performed a pooled analysis of published literature from Sweden, Denmark, and Finland and noted that DM was associated with an increased risk of developing a wide array of cancers, including breast, ovarian, lung, pancreatic, stomach, colorectal, and non-Hodgkin lymphoma[Citation7]. Since 1916, it has been speculated that patients with PM have a similarly increased risk of developing cancer. Cancers commonly associated with PM include breast [Citation8–10], squamous cell lung cancer [Citation11], adenocarcinoma of lung [Citation12–14], bladder cancer, and Non-Hodgkin lymphoma [Citation1,Citation8,Citation15]. Additionally, literature review reveals an isolated case of PM associated with colon cancer[Citation16]. Based on literature review, inflammatory myopathies, such as PM, are rarely associated with pancreatic neoplasms[Citation7].
2.3. Clinical presentation
Pancreatic cancer-associated myopathy remains a rare syndrome. A meta-analysis of 20 studies performed by Yang et al. reviewed cancer-associated myopathies occurring in various malignancies and only 3/20 studies implicated pancreatic cancer[Citation17]. Of the 95 PM patients reviewed by Hill et al. who developed cancer, there was only one case of pancreatic cancer[Citation7]. The other two studies had 33 and 43 patients, respectively, and each had just one case of pancreatic cancer [Citation18,Citation19]. Kida et al., Syrios et al., Siddiqui et al., and Amroun et al. have also reported isolated cases of pancreatic adenocarcinoma associated with PM as described in [Citation15,Citation20–22]. Two of four patients received treatment with Gemcitabine, a known cause of drug-induced myopathy [Citation23,Citation24]. Chemotherapeutic agents typically cause myopathies to sites previously exposed to radiation, but gemcitabine is unique in that it can cause myopathies in radiation-naïve patients[Citation25]. Therefore, administration of chemotherapeutic gemcitabine further complicates diagnosis of cancer-associated PM or myopathy.
Table 1. Cases of pancreatic carcinoma associated polymyositis and myopathy
PM characteristically affects the proximal muscles of the upper and lower extremities. Lumbar MRI in our case revealed edema within the paraspinal and psoas muscles, suggesting myopathy is consistent with PM affecting these muscle groups (). While imaging in PM is more commonly performed in the proximal lower extremities, specifically the thighs, whole-body imaging with PET or MRI suggests that involvement of paraspinal and psoas muscle groups is not uncommon. Whole-body MRI performed on 129 patients with PM or DM revealed muscle inflammation in 105 patients. Inflammation of pelvic and lumbar musculature was noted in 94 and 85 patients respectively[Citation26]. O’Connell et al. reported involvement of the psoas in four out of seven patients with PM reviewed. [Citation27] Involvement of paraspinal and psoas musculature is likely rarely reported due to conventional imaging practices. Our case demonstrates that paraspinal and psoas musculature may be implicated in paraneoplastic PM.
2.4. Diagnosis and pathology
DM or PM are typically diagnosed with muscle biopsy findings. DM can also be diagnosed by skin biopsy or by the combination of proximal muscle weakness and the presence of a characteristic rash. EMG and auto-antibodies may further support the diagnosis of PM or DM but are not required for diagnosis.
Our patient was diagnosed with paraneoplastic myopathy because of symmetrical, proximal muscle weakness, elevated CK level, imaging findings, and response to steroids in the setting of a new pancreatic adenocarcinoma diagnosis. While a muscle biopsy would have solidified the diagnosis of paraneoplastic myopathy, this was unfortunately not obtained.
Statin-induced myopathy (SIM) was considered on the initial differential and statin therapy was discontinued upon arrival at the hospital. SIM was felt to be less likely, as the symptoms did not start upon initiation and persisted despite discontinuation of the statin. Immune mediated necrotizing myopathy (IMNM) was also considered. IMNM typically presents as a subacute progressive myopathy with presence of HMGCR autoantibodies[Citation28]. Statin therapy can rarely result in immune mediate necrotizing myopathy. Although less likely, without a biopsy and antibody panel, we cannot definitively rule this out. To further elucidate similar cases, muscle biopsy, auto-antibodies and electromyography studies should be pursued. However, the new diagnosis of pancreatic cancer, lack of rash to support the diagnosis of DM, and response to steroids make paraneoplastic myopathy the most likely etiology in our case.
2.5. Treatment
Paraneoplastic PM is treated similarly to idiopathic PM. Both conditions are typically treated with high-dose corticosteroids followed by a transition to a steroid-sparing agent such as methotrexate or azathioprine [Citation2,Citation29]. Response to therapy for DM and PM ranges from weeks to months [Citation2,Citation29,Citation30]. Our patient had a rather prompt response to therapy, which is not typical for DM or PM but has been reported in the literature with myopathies [Citation30–32].
It is notable that paraneoplastic PM is less likely to respond to steroid therapy and lack of expected response to steroids should prompt evaluation of underlying malignancy. Treatment of the associated cancer results in improvement of myositis [Citation6,Citation33]. Paraneoplastic PM significantly worsens the overall prognosis of recovery of muscle function as compared to other cases of PM [Citation1,Citation34–36].
3. Conclusion
This case exemplifies the importance of maintaining clinical suspicion of underlying malignancies in patients presenting with symptoms of inflammatory myopathies, such as DM and PM. It is crucial to perform thorough evaluation of malignancy as early diagnosis and treatment may improve prognosis specifically in cases of DM and PM. Concurrent rhabdomyolysis is also important to consider when diagnosing PM. Chemotherapeutic agents, such as gemcitabine, have been implicated in myopathies and should be noted on medication survey in addition to statin therapies. Atypical muscle group involvement does not rule out PM. Ultimately, muscle biopsy, serum antibodies, and EMGs should be used in making a definitive diagnosis of paraneoplastic myopathy, PM, and DM.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Sigurgeirsson B, Lindelöf B, Edhag O, et al. Risk of cancer in patients with dermatomyositis or polymyositis. A population-based study. N Engl J Med. 1992;326(6):363–367.
- Pelosof LC, Gerber DE. Paraneoplastic syndromes: an approach to diagnosis and treatment. Mayo Clin Proc. 2010;85(9):838–854.
- Zampieri S, Valente M, Adami N, et al. Polymyositis, dermatomyositis and malignancy: a further intriguing link. Autoimmun Rev. 2010;9(6):449–453.
- Meyer A, Meyer N, Schaeffer M, et al. Incidence and prevalence of inflammatory myopathies: a systematic review. Rheumatology (Oxford). 2015;54(1):50–63.
- Tan JA, Roberts-Thomson PJ, Blumbergs P, et al. Incidence and prevalence of idiopathic inflammatory myopathies in South Australia: a 30-year epidemiologic study of histology-proven cases. Int J Rheum Dis. 2013;16(3):331–338.
- Ponyi A, Constantin T, Garami M, et al. Cancer-associated myositis: clinical features and prognostic signs. Ann N Y Acad Sci. 2005;1051:64–71.
- Hill CL, Zhang Y, Sigurgeirsson B, et al. Frequency of specific cancer types in dermatomyositis and polymyositis: a population-based study. Lancet. 2001;357(9250):96–100.
- Croce S, Guèye M, Korganow AC, et al. Paraneoplastic polymyositis associated with breast cancer: a therapeutic emergency. Breast Cancer Res Treat. 2011;126(3):811–814.
- Merali N, Yousuff M, Pronisceva V, et al. Paraneoplastic polymyositis presenting as a clinically occult breast cancer. Ann R Coll Surg Engl. 2017;99(2):e40–e43.
- Dias LP, Faria AL, Scandiuzzi MM, et al. A rare case of severe myositis as paraneoplastic syndrome on breast cancer. World J Surg Oncol. 2015;13:134.
- Gabrilovich M, Raza M, Dolan S, et al. Paraneoplastic polymyositis associated with squamous cell carcinoma of the lung. Chest. 2006;129(6):1721–1723.
- Acciavatti A, Avolio T, Rappuoli S, et al. Paraneoplastic necrotizing myopathy associated with adenocarcinoma of the lung - a rare entity with atypical onset: a case report. J Med Case Rep. 2013;7:112.
- Fujita J, Tokuda M, Bandoh S, et al. Primary lung cancer associated with polymyositis/dermatomyositis, with a review of the literature. Rheumatol Int. 2001;20(2):81–84.
- Uchibori K, Ogata T, Shirai T, et al. Lung cancer diagnosed more than five years after the development of polymyositis/dermatomyositis. ISRN Pulmonol. 2013;2013:1–6.
- Syrios J, Kechagias G, Xynos ID, et al. Pancreatic adenocarcinoma-associated polymyositis treated with corticosteroids along with cancer specific treatment: case report. BMC Gastroenterol. 2011;11:33.
- Rominiyi O, Broman DM, Rajaganeshan R, et al. Colon cancer presenting with polymyositis-A case report. Int J Surg Case Rep. 2011;2(7):225–227.
- Yang Z, Lin F, Qin B, et al. Polymyositis/dermatomyositis and malignancy risk: a metaanalysis study. J Rheumatol. 2015;42(2):282–291.
- Chen YJ, Wu CY, Huang YL, et al. Cancer risks of dermatomyositis and polymyositis: a nationwide cohort study in Taiwan. Arthritis Res Ther. 2010;12(2):R70.
- Buchbinder R, Forbes A, Hall S, et al. Incidence of malignant disease in biopsy-proven inflammatory myopathy. A population-based cohort study. Ann Intern Med. 2001;134(12):1087–1095.
- Kida Y, Maeshima E, Furukawa K, et al. A case of polymyositis with a significantly high level of KL-6 associated with pancreatic cancer. Mod Rheumatol. 2007;17(3):262–264.
- Siddiqui S, Fiorillo M, Tzeng J, et al. Polymyositis as a paraneoplastic phenomenon of occult pancreatic cancer. Am J Gastroenterol. 2011;106:S220.
- Amroun KL, De Mestier L, Deguelte-Lardiere S, et al. Pancreas cancer-associated polymyositis of the legs regressing after cephalic duodenopancreatectomy: case report and review of the literature. JOP. 2012;13(6):674–676.
- Spielmann L, Messer L, Moreau P, et al. Gemcitabine-induced myopathy. Semin Arthritis Rheum. 2014;43(6):784–791.
- Burris HA 3rd, Hurtig J. Radiation recall with anticancer agents. Oncologist. 2010;15(11):1227–1237.
- Ardavanis AS, Ioannidis GN, Rigatos GA. Acute myopathy in a patient with lung adenocarcinoma treated with gemcitabine and docetaxel. Anticancer Res. 2005;25(1B):523–525.
- Huang ZG, Gao BX, Chen H, et al. An efficacy analysis of whole-body magnetic resonance imaging in the diagnosis and follow-up of polymyositis and dermatomyositis. PloS One. 2017;12(7):e0181069.
- O’Connell MJ, Powell T, Brennan D, et al. Whole-body MR imaging in the diagnosis of polymyositis. AJR Am J Roentgenol. 2002;179(4):967–971.
- Shovman O, Gilburd B, Chayat C, et al. Anti-HMGCR antibodies demonstrate high diagnostic value in the diagnosis of immune-mediated necrotizing myopathy following statin exposure. Immunol Res. 2017;65(1):276–281.
- Joffe MM, Love LA, Leff RL, et al. Drug therapy of the idiopathic inflammatory myopathies: predictors of response to prednisone, azathioprine, and methotrexate and a comparison of their efficacy. Am J Med. 1993;94(4):379–387.
- Ghate J, Katsambas A, Augerinou G, et al. Review article: a therapeutic update on dermatomyositis/polymyositis. Int J Dermatol. 2000;39(2):81–87.
- Bronner IM, Hoogendijk JE, Wintzen AR, et al. Necrotising myopathy, an unusual presentation of a steroid-responsive myopathy. J Neurol. 2003;250(4):480–485.
- van Dijk S, van der Kooi AJ, Aronica E, et al. Paraneoplastic necrotizing autoimmune myopathy in a patient undergoing laparoscopic pancreatoduodenectomy for distal cholangiocarcinoma. Case Rep Gastroenterol. 2016;10(3):525–530.
- András C, Ponyi A, Constantin T, et al. Dermatomyositis and polymyositis associated with malignancy: a 21-year retrospective study. J Rheumatol. 2008;35(3):438–444.
- Carpenter JR, Bunch TW, Engel AG, et al. Survival in polymyositis: corticosteroids and risk factors. J Rheumatol. 1977;4(2):207–214.
- Aggarwal R, Oddis CV. Paraneoplastic myalgias and myositis. Rheum Dis Clin North Am. 2011;37(4):607–621.
- Maugars YM, Berthelot JM, Abbas AA, et al. Long-term prognosis of 69 patients with dermatomyositis or polymyositis. Clin Exp Rheumatol. 1996;14(3):263–274.