ABSTRACT
Circulating extracellular vesicles (EVs) such as cholera toxin B chain (CTB)- or annexin V (AV)-binding EVs were previously shown to be rich sources of biomarkers. Here we test if previously identified pre-eclampsia (PE) candidate biomarkers, TIMP-1 in CTB-EVs (CTB-TIMP) and PAI-1 in AV-EVs (AV-PAI) complement plasma PlGF in predicting PE in a low-risk obstetric population. Eight hundred and forty-three prospectively banked plasma samples collected at 28 + 0 to 32 + 0 gestation weeks in the Neonatal and Obstetrics Risk Assessment (NORA) cohort study were assayed by sandwich ELISAs for plasma PlGF, CTB-TIMP1 and AV-PAI1. Nineteen patients subsequently developed PE 7.3 (±2.9) weeks later at a mean gestational age of 36.1 ± 3.5 weeks. The biomarkers were assessed for their predictive accuracy for PE using stepwise multivariate logistic regression analysis with Firth correction and Areas under the curve (AUC). To achieve 100% sensitivity in predicting PE, the cut-off for plasma PlGF, CTB-TIMP1 & AV-PAI1 were set at <1235, ≤300 or >1300 and <10,550 pg/mL plasma, respectively. The corresponding AUCs, specificity and PPV at a 95% confidence interval were 0.92, 52.1% and 4.7%; 0.72, 44.5% and 4.0%; and 0.69, 21.5% and 2.9%, respectively. At 100% sensitivity, the three biomarkers had a combined AUC of 0.96, specificity of 78.6%, and PPV of 9.9%.
This is the first large cohort validation of the utility of EV-associated analytes as disease biomarkers. Specifically, EV biomarkers enhanced the predictive robustness of an existing PE biomarker sufficiently to justify PE screening in a low-risk general obstetric population.
Responsible Editor Yong Song Gho, Pohang University of Science and Technology Life Sciences, Republic of Korea
Introduction
Pre-eclampsia (PE) is a significant pregnancy-related complication in both developed and developing countries with an incidence rate of 4–18% of all deliveries [Citation1]. Although PE is long recognised as the diseased manifestation of a dysfunctional placenta, the molecular pathogenesis of PE has not been elucidated, making PE poorly tractable to therapeutic intervention. Nevertheless, early detection with timely implementation of appropriate clinical support could improve outcome for both mother and child. However, most of PE patients are not identified early enough to provide sufficient time window for effective clinical management to delay or alleviate the pathological manifestations of the disease.
To enhance discovery of robust predictive PE biomarkers, we had previously proposed targeting specific classes of plasma extracellular vesicle (EV) instead of whole plasma for PE biomarker discovery [Citation2]. EV is a collective term describing all classes of secreted bi-lipid membrane vesicles such as exosomes, microvesicles, ectosomes, membrane particles, exosome-like vesicles, apoptotic bodies, prostasome or oncosomes. Many different cell types are known to secrete EVs to mediate intercellular communication by delivering their cargo of protein or RNA from secreting cells to recipient cells [Citation3]. Therefore, their cargos are sensitive sentinels of dynamic disease-induced cellular changes, and class- or cell-specific EVs are more likely to yield potent disease biomarkers. There is currently no definitive protocol to isolate class- or cell-specific EVs. To circumvent this, we had proposed isolating different EV classes by targeting rare membrane lipids such as GM1 ganglioside and phosphatidylserine through their high affinity for cholera toxin B chain (CTB) and annexin V (AV) respectively [Citation4,Citation5]. We have reported that plasma CTB-binding EVs (CTB-EVs) and AV-binding EVs (AV-EVs) from pregnant women with or without pre-eclampsia represent unique EV classes with different permutations of candidate biomarkers for PE [Citation2]. Several candidate PE biomarkers, namely Procalcitonin (PCT), Endoglin/CD105 (ENG), Growth Hormone (GH), Tissue Inhibitor Of Metalloproteinases 1 (TIMP1), Plasminogen Activator Inhibitor; Type I (PAI1), Placental Growth Factor (PlGF), S-100b Transforming Growth Factor beta (TGF-β) and soluble fms-like tyrosine kinase-1 (sFlt-1) were present in plasma CTB-EVs, AV-EVs or both.
In this study, we aimed to identify EV-associated biomarkers that could either singly or in combination predict PE in a low-risk general obstetric population.
Methods
NORA cohort study and plasma collection
The NORA study is a prospective cohort study of 926 women with viable, singleton pregnancies seeking antenatal care and delivering within a single hospital [Citation6]. It was conducted to identify clinical and biochemical risk factors associated with the various adverse pregnancy outcomes. Pregnancies with multiple pregnancies, diagnosed aneuploidy or fetal anomaly, and pregnant women with autoimmune or renal disease, were excluded from the study. Plasma among other bio-samples were collected at four gestation stages, 11 to 14, 18 to 22, 28 to 32 and >34 gestation weeks. The study was approved by the Centralised Institutional Review Board (CIRB) and informed written consent was obtained from each participant. Of the 926 women registered in the cohort, plasma from 843 women collected at 28 to 32 gestation weeks (i.e. visit 3) were used in this study as some patients missed their appointments or some of the samples were not available. Nineteen (2.2%) of 843 patients developed PE. About 8 mL of blood are drawn from consented subject in both EDTA and clear blood tubes. It is then centrifuge on a table top centrifuge machine at 2000rpm for 15 minutes before storing the plasma in 1 mL cryovials at −80 °C freezer.
Diagnosis of pre-eclampsia
Preeclampsia is diagnosed when a pregnant woman develops blood pressure ≥ 140 mm Hg systolic or ≥ 90 mm Hg diastolic on two separate readings taken at least 4 to 6 hours apart after 20 weeks gestation in an individual with previously normal blood pressure and coupled with proteinuria ≥ 0.3 grams of protein in a 24-hour urine sample or a urine dipstick reading of 1+ or greater. A severe preeclampsia is diagnosed when blood pressure ≥ 160 mm Hg systolic or ≥ 110 mm Hg diastolic with proteinuria > 5 grams of protein in a 24-hour urine sample or a urine dipstick reading of 3+ or greater.
Isolation of CTB-EVs and AV-EVs
CTB-EVs and AV-EVs were isolated from plasma as previously described [Citation2]. Briefly, 30 μL plasma and 150 ηg biotinylated CTB or 150 ηg biotinylated AV were incubated in 100 μL Binding Buffer (0.1M Hepes, 1.4M NaCl, and 25 mM CaCl2) for 30 minutes at 37°C in a rotating tube. Twenty μL of washed Streptavidin Coated Polystyrene Particles (Spherotech) in the Binding Buffer were then added to the reaction mix. The beads and reaction mix were incubated at 37°C with rotation for another 30 minutes before being transferred to a 1.2 μm AcroPrep Supor Pall filter plate (Pall). The binding buffer was removed by vacuum filtration and the beads were washed thrice with 200 μL Binding Buffer. The EVs were then lysed in 200 μL of Lysis Buffer (Biovision). The lysed EVs were then assayed by ELISA.
Enzyme link immunosorbent assay for PAI1, PlGF and TIMP1
GH, PCT, PAI1, PlGF and TIMP1 concentrations were determined by commercially available sandwich ELISA kits (RayBiotech) according to the manufacturer’s instructions. For each biomarker 200 μL of the lysed EVs (as described above) or 30 μL of plasma diluted to 200 μL with dilution buffer were incubated with the respective immobilised primary antibody at 4°C overnight. After three washes, the horseradish peroxidase (HRP)-conjugated secondary antibodies were added and incubated for 3 hours at room temperature. After three washes, tetramethylbenzidine (TMD) solution was added and incubated for 45 minutes. The reaction was stopped by the addition of the acidic “stop” solution provided by the manufacturer and absorbance of the resulting yellow product was measured at λ450 nm, using a Tecan microplate reader. Concentrations were determined from a standard curve generated by the standards provided in the kit.
Statistical analysis
Statistical analysis was performed using SAS 9.4 (SAS Inc., Cary, NC, USA) and STATA 13 (Statacorp, College Station, TX, USA). The Pearson chi-square/Fisher’s exact test were used to compare the control and pre-eclampsia study groups for race and socio-clinical categorical variables. Similarly, either the two-sample t-test or Wilcoxon rank-sum test was applied, as appropriate, for continuous variables. Univariate logistic regression was performed on baseline variables to screen for candidate predictors. Variables significant at p < 0.20 in the univariate analysis were included in the selection list of a stepwise multiple logistic regression (sig. levels to enter and stay, SLE = 0.20 and SLS = 0.25). Ultimately, the rationale for selection of variables for inclusion into the final predictive model was not strictly statistical, but was predicated upon both statistical and clinical considerations. A receiver operating characteristic (ROC) curve was obtained for each selected variable arising from a univariate logistic regression analysis. In addition, a ROC curve was obtained from the multiple logistic regression analysis incorporating all of the selected variables. Because the predictive model will be used to screen patients at high risk of pre-eclampsia, cut-points were identified to provide 100% sensitivity, and hence 100% NPV. P-values ≤0.05 were considered statistically significant.
Results
Validation of plasma PlGF, CTB-TIMP1 and AV-PAI1 as candidate PE biomarkers
We had previously reported using plasma from patients at 28–38 weeks gestation and antibody arrays that the concentration of PCT, ENG, GH, TIMP1, PAI1, PlGF, S-100b, TGF-β and sFLT-1 in CTB- and AV-EVs from PE patients was significantly different from those of healthy controls [Citation2]. Of these candidate biomarkers, PCT, GH, TIMP1, PAI1 and PlGF were further evaluated using the more robust and sensitive quantitative sandwich ELISA kits. Here we used prospectively banked plasma samples collected at 34–36 weeks of gestation from participants in the NORA study cohort. Plasma samples from 10 pre-eclampsia and 20 matched healthy pregnant patients were extracted for CTB-EV and AV-EVs. The candidate biomarkers in the plasma, plasma CTB-EV and plasma AV-EV of each patient or control were then assayed ( and ). Notably, each biomarker was differently distributed in the plasma and the two EV types i.e. each biomarker has three permutations and only one permutation could serve as a biomarker. There was no difference in GH and PCT levels between control and PE patients in the plasma, CTB- and AV-EV ( and ). In contrast, CTB-TIMP1, and plasma PlGF were significantly lower in PE patients (P-Value <0.05) while AV-PAI1 were significantly higher in PE patients (P-Value <0.05) ( and ).
Figure 1. a, b, Concentration of GH, PCT, in PE and healthy pregnant women. Prospectively banked plasma samples collected at 34–36 weeks of gestation from 10 pre-eclampsia and 20 matched healthy pregnant patients were assayed for Growth Hormone (GH) and Procalcitonin (PCT), in the plasma, plasma CTB-EV and plasma AV-EVs.
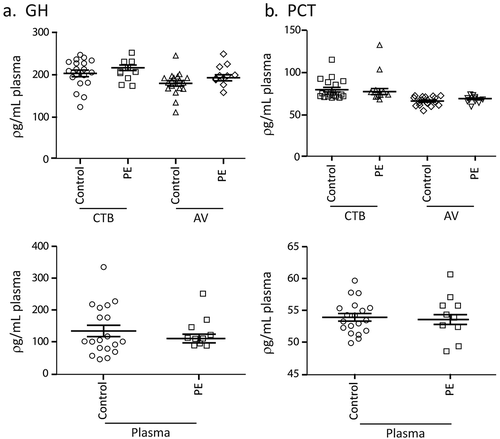
Figure 2. a, b, c Concentration of PAI1, PIGF, TIMP1 in PE and healthy pregnant women. Prospectively banked plasma samples collected at 34–36 weeks of gestation from 10 pre-eclampsia and 20 matched healthy pregnant patients were assayed for Plasminogen Activator Inhibitor; Type I (PAI1), Placental Growth Factor (PlGF), and Tissue Inhibitor Of Metalloproteinases 1 (TIMP1) in the plasma, plasma CTB-EV and plasma AV-EVs.
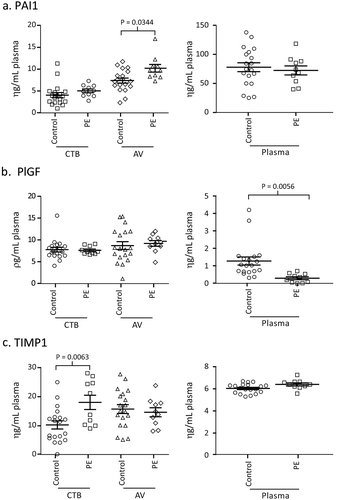
Figure 3. a, b, c Concentration of PAI1, PlGF and TIMP1 in healthy pregnant women of 3 different races. The concentration of PAI1, TIMP1, and PlGF in plasma, plasma CTB-EV and plasma AV-EVs from 20 Chinese, 20 Malay and 20 Indian healthy pregnant patients at 34–39 weeks of gestation were assayed.
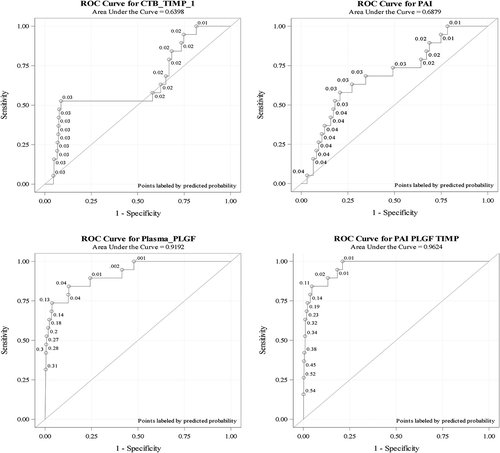
Figure 4. ROC curves for CTB-TIMP, AV-PAI and plasma PlGF. Univariate and multivariate receiver operating characteristic (ROC) curves and the corresponding area under the curve (AUC) were calculated for CTB-TIMP, AV-PAI, plasma PlGF and the combination of all three biomarkers by presetting sensitivity at 100%.
PAI1, PlGF and TIMP1 levels were independent of races
The Singapore population has three major racial groups, Chinese, Malay and Indians. The Malay race has been associated with a higher incidence of PE and eclampsia [Citation7] suggesting that race could be a confounding factor in a PE candidate biomarker. To assess this possibility, the concentration of CTB-TIMP, AV-PAI and plasma PlGF was assayed using plasma samples from the NORA study. 20 Chinese, 20 Malay and 20 Indian healthy pregnant patients between 34–39 weeks of gestation were tested. There are no significance differences in the level of CTB-TIMP, AV-PAI and plasma PlGF among the three races (p values > 0.05) indicating that the levels of these candidate biomarkers are race independent ().
Statistical analysis of CTB-TIMP, AV-PAI and plasma PlGF as predictive PE biomarkers
The robustness of CTB-TIMP, AV-PAI and plasma PlGF as PE biomarkers were next evaluated using the 843 plasma samples that were prospectively banked at 28–32 weeks gestation in the NORA study cohort. Characteristics of this study population were summarized in . Of the 843 patients, 19 eventually developed PE. There were no statistically significant differences between the control and PE groups in terms of maternal age, race composition, parity, gravida, education and incidence of IUGR. The BMI, average SBP and DBP, each of the three biomarkers and incidence of preterm delivery were significantly different between patients who subsequently developed PE and those who did not (P-Value <0.05). The mean interval from Visit 3 when plasma was collected for this study to subsequent PE diagnosis was 7.3 (±2.9) weeks with a range from 1.29 weeks to 11.24 weeks. Two patients were diagnosed at 32 weeks after plasma was taken, one of whom was diagnosed on the same day after the plasma sample was taken. All the other PE patients were diagnosed at or after 34 weeks at a mean gestational age of 36.1 (±3.5) and they delivered at a mean gestational age of 36.7 (±3.2). In contrast, the controls delivered at a mean gestational age of 38.8 (+1.3).
To provide for a 100% sensitivity, the cut-off points for plasma PlGF, CTB-TIMP and AV-PAI were set at <1235, ≤300 or >1300 and <10,550 ρg/mL, respectively. At 100% sensitivity, the area under the ROC (AUC) for each of the three biomarkers, AV-PAI, CTB-TIMP, plasma PIGF and the combination of all three biomarkers were 0.69, 0.72, 0.92 and 0.96, respectively (, ). The corresponding specificities were 21.5%, 44.5%, 52.1% and 78.6% (). At a prevalence of 2.3%, the corresponding PPVs were 2.9, 4.0, 4.7 and 9.9.
Table 1. Profile summary of non-PE controls and PE patients.
Table 2. Predictive performance of biomarkers.
Discussion
This study demonstrated that EV-based biomarkers, CTB-TIMP and AV-PAI could enhance the sensitivity and specificity of PlGF, a widely used biomarker for PE prediction in commercial kits such as Triage PlGF test, Elecsys immunoassay sFlt-1/PlGF ratio, DELFIA Xpress PlGF 1–2-3 test, and BRAHMS sFlt-1 Kryptor/BRAHMS PlGF plus Kryptor PE ratio. However, these kits do not have the sensitivity or specificity to screen for PE in low-risk obstetric population. Both the American College of Obstetricians and Gynecologists (ACOG) Committee on Obstetric Practice in their Committee Opinion No.638, Sept 2015 (reaffirmed 2017; https://www.acog.org/-/media/Committee-Opinions/Committee-on-Obstetric-Practice/co638.pdf?dmc=1&ts=20170803T0750324402) (supplementary data 1) and the National Institute for Health and Care Excellence (NICE) Diagnostics guidance 23, 11 May 2016 (https://www.nice.org.uk/guidance/dg23) (supplementary data 2) recommended detailed medical history as the best screening approach for PE. While ACOG recommended against the use of PE biomarkers to predict PE, NICE recommended that the Triage PlGF test and the Elecsys immunoassay sFlt 1/PlGF ratio be used only to rule-out but not rule-in PE in women presenting with suspected PE between 20 weeks and 34 weeks of gestation. Collectively, the ACOG and NICE recommendations highlighted the critical need for biomarkers to predict PE in the low-risk general obstetric population and a need to rethink the current approach in plasma/serum biomarker discovery.
Plasma and serum are ideal for biomarker discovery as they are the main conduits for the secretion and extraction of proteins by normal and diseased cells and have proteome complexity of > 10,000 proteins. However, >99% proteins are highly abundant proteins such as albumin, IgG, transferrin (Schuh, Bruxel et al. 2014) making discovery of plasma protein disease biomarker exceedingly rare and difficult. Recently, plasma was found to be rich in protein- and RNA-containing extracellular vesicles (EVs) i.e. 1012 particles per mL (Li, Zeringer et al. 2014). As EVs are thought to mediate intercellular communication, they are more sensitive sentinels of the dynamic disease-induced cellular changes and are therefore enriched in disease-relevant biomarkers. Additionally, using plasma EV instead of plasma would reduce noise to signal ratio caused by high abundance plasma proteins and enable discovery of biomarkers.
As EV is a collective term that describes all classes of secreted lipid membrane vesicles such as microvesicles, ectosomes, exosomes etc and plasma EVs are likely to be secreted by many different cell types, a specific EV class from a specific cell source is more likely to yield potent disease biomarkers. However, plasma EVs are routinely isolated according to size or density by centrifugation, filtration or precipitation [Citation8,Citation9]. Since the size and density of EVs overlapped significantly across several classes, size- or density-based isolation techniques essentially enriched for large complexes including all EV types and large protein or lipoprotein complexes. While immuno-isolation techniques using antibodies against specific membrane proteins could increase the specificity of EV isolation, they also have several limitations. For example, no EV class or cell type has been defined by a single membrane protein and antibodies against membrane proteins cannot distinguish between membrane vesicles, protein complexes or soluble receptors. As described in the introduction, we had circumvented this challenge in isolating EV types by targeting rare membrane lipids with lipid-binding proteins to ensure that only complexes with membrane lipids are extracted. As discussed in our previous study [Citation2], these complexes would have to be vesicles as they are the thermodynamically configuration of bi-polar membrane lipids in aqueous physiological fluids. This feature and the presence of membrane proteins such as the tetraspanins in both CTB or AV isolates provide supporting evidence that the CTB- and AV-EVs as described here are bona fide EVs. Recently, we have also observed by cryo EM that AV-EVs isolated from ascites are bi-lipid membrane vesicles [Citation10]. In addition, we have previously shown by scanning electron microscopy that CTB and AV extract ~100 ηm vesicles and these different EV types carried different permutations of proteins and RNA [Citation2,Citation4]. In this study, we observed that TIMP-1 in only CTB-EVs and PAI-1 in AV-EVs were significantly different between PE and healthy pregnant women. TIMP-1 and PAI-1 were reported to play critical roles in the placenta formation and establishment of the maternal-fetal circulation [Citation11]. However, plasma TIMP-1 and PAI-1 despite a long association with PE [Citation12] have not developed into PE biomarkers. Our observation that CTB-TIMP and AV-PAI but not plasma/AV-TIMP and plasma/CTB-PAI are robust PE biomarkers suggests that the contribution of TIMP-1 and PAI-1 to pathogenesis of PE depend on the vehicular support provided by different EV types, and that the EV context is an important consideration in the disease pathology. Although both CTB-TIMP and AV-PAI in this study only complement an established plasma-based biomarker, their significant enhancement of the predictive robustness of plasma PlGF nonetheless provides a rationale for an EV role in discovery of novel biomarkers or re-discovery of established plasma biomarkers in a novel permutation i.e. plasma EVs.
This is the first cohort validation of the utility of EV-associated analytes as predictive biomarkers of PE. Specifically, we demonstrated that in a low-risk population, the biomarker trio of plasma PlGF, CTB-TIMP and AV-PAI predicted PE in patients at a mean 7.3 ± 2.9 weeks before diagnosis with an AUC (95% CI) of 0.96 (0.93–0.99) at 100% sensitivity, 78.6% specificity, 9.9% PPV at and 100% NPV. CTB-TIMP and AV-PAI enhanced the robustness of PlGF as a PE predictive biomarker with an increase in AUC from 0.92 to 0.96, 110% increase in PPV from 4.7% to 9.9% and a 50% increase in specificity from 52.1% to 78.6%. In contrast, one of the two PlGF-based test kits recommended by NICE to rule-out but not rule-in PE in women, the Elecsys immunoassay has a NPV of 99.3% for ruling out preeclampsia within 1 week in a high-risk population and PPV of 36.7% for ruling in PE in 4 weeks [Citation13]. The low 9.9% PPV of our biomarker trio is due primarily to a low baseline risk of 2.3 % versus 21.7% in the Zeisler et al. study. Nevertheless, the 9.9% PPV of our biomarker trio represents a 4.3 fold increase above baseline compared to a 1.7 fold increase in the latter study.
In summary, this study showed that the biomarker trio which was tested independently of clinical presentation could be applied as an independent diagnostic aid to identify patients at risk in a low-risk general obstetric population or to predict risk in patients suspected of PE. This predictive potency could also be further enhanced by factoring in blood pressure and proteinuria. However, this would require further clinical validation with a larger random cohort.
Supplemental_data.docx
Download MS Word (13 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary Material
Supplemental data for this article can be accessed here.
Additional information
Funding
Notes on contributors
Sai Kiang Lim
SKL and KHT conceptualised and wrote the manuscript; SST and WKS performed the assays and were responsible for –; MJN and KHT set up the NORA cohort study; WST and MJN were responsible for and ; WST and JCA did the statistical analysis. All authors reviewed the manuscript.
References
- Villar J, Say L, Gulmezoglu AM, et al. Eclampsia and pre-eclampsia: a health problem for 2000 years. In: Critchly H, MacLean A, Poston L, et al., editors. Pre-eclampsia. London: RCOG Press; 2003. p. 189–9.
- Tan KH, Tan SS, Sze SK, et al. Plasma biomarker discovery in preeclampsia using a novel differential isolation technology for circulating extracellular vesicles. Am J Obstet Gynecol. 2014 Oct;211(4):380.e1–380.e13.
- Yáñez-Mó M, Siljander PR-M, Andreu Z, et al. Biological properties of extracellular vesicles and their physiological functions [physiology]. J Extracell Vesicles. 2015;4:27066.
- Lai RC, Tan SS, Yeo RWY, et al. MSC secretes at least 3 EV types each with a unique permutation of membrane lipid, protein and RNA. J Extracell Vesicles. 2016;5:29828.
- Tan SS, Yin Y, Lee T, et al. Therapeutic MSC exosomes are derived from lipid raft microdomains in the plasma membrane. J Extracell Vesicles. 2013;2. [pii]. PubMed PMID: 24371518; PubMed Central PMCID: PMC3873122. eng. DOI:10.3402/jev.v2i0.22614.
- Lim WY, Saw SM, Tan KH, et al. A cohort evaluation on arterial stiffness and hypertensive disorders in pregnancy. BMC Pregnancy Childbirth. 2012;12:160.
- Tan KH, Kwek K, Yeo GS. Epidemiology of pre-eclampsia and eclampsia at the KK Women’s and Children’s Hospital, Singapore. Singapore Med J. 2006 Jan;47(1):48–53. PubMed PMID: 16397721; eng.
- Lobb RJ, Becker M, Wen Wen S, et al. Optimized exosome isolation protocol for cell culture supernatant and human plasma [exosomes; cancer; plasma; cell culture; isolation; purification]. J Extracell Vesicles. 2015;4:27031.
- Witwer KW, Buzás EI, Bemis LT, et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research [Extracellular vesicle; RNA; isolation; characterization; biomarker; intercellular communication; biofluid; immunology; methodology]. J Extracell Vesicles. 2013;2:20360.
- Reiner AT, Tan S, Agreiter C, et al. EV-associated MMP9 in high-grade serous ovarian cancer is preferentially localized to annexin V-binding EVs. Dis Markers. 2017;2017:1–9.
- Zhu J-Y, Pang Z-J, Yu Y-H. Regulation of trophoblast invasion: the role of matrix metalloproteinases. Rev Obstet Gynecol. 2012;5(3–4):e137–e143.
- Kolben M, Lopens A, Bläser J, et al. Proteases and their inhibitors are indicative in gestational disease. Eur J Obstetrics Gynecol Reprod Biol. 1996;68:59–65.
- Zeisler H, Llurba E, Chantraine F, et al. Predictive value of the sFlt-1: plGFRatio in women with suspected preeclampsia. New Engl J Med. 2016;374(1):13–22.
