ABSTRACT
Extracellular vesicles (EVs) deliver biologically active cargos from donor cells to recipient cells for intercellular communication. Since the existence of RNA cargo was discovered, EVs have been considered to be useful drug-delivery systems. Specifically, EVs from bovine milk (mEV) are one of the most promising platforms, since bovine milk is a scalable source of EVs for mass production. However, it is still difficult to isolate pure EVs from bovine milk owing to the complexity of raw materials. Furthermore, the biocompatibility and immunotoxicity of mEVs are still unclear. In this study, we developed a new method for isolating bovine milk-derived EVs by employing acid treatment and ultracentrifugation. Isolated mEVs are spherical in shape, measure 120 nm in diameter and contain typical EV marker proteins, such as tetraspanins. Compared with the previously reported method, our method can isolate purer mEVs. When mEVs are contacted with the mouse macrophage cell line Raw264.7, mEVs are readily taken up by the cells without a cytotoxic effect, suggesting that mEVs can deliver the cargo molecules into cells. While systemic administration of mEVs into mice resulted in the absence of systemic toxicity, certain types of cytokines were slightly induced. No anaphylaxis effect was observed after serial administration of mEVs in mice. Thus, mEVs isolated using our method are well tolerated in vivo and may be useful for the drug-delivery application.
Introduction
Extracellular vesicles (EVs) are membranous nanoparticles secreted from living cells. EVs include exosomes, microvesicles and other types of secreted vesicles [Citation1]. It has been discovered that EVs contain RNA and exchange genetic information between the cells [Citation2,Citation3]. Since then, EVs have been expected to be used as a natural drug-delivery system (DDS) that enables intracellular delivery of nucleic acids to specific cells [Citation4–Citation6]. For example, exosomes derived from dendritic cells were modified by targeting peptide and used for the delivery of short interfering RNA (siRNA) into mouse brain [Citation7]. Ohno et al. achieved the delivery of anti-tumour microRNA (miRNA) to cancer cells in vitro and in vivo using exosomes from the HEK293 cells [Citation8]. However, the yield of EVs from the conditioned medium is insufficient for clinical use. Although some researchers developed a culture system to collect large amounts of EVs from cultured cells [Citation9], the yield of EVs was still low. Furthermore, production of EVs from the conditioned medium relies on laborious and expensive cell-culture steps. Difficulty of quality control of cell culture and its products is another issue for the production of EVs from the conditioned medium. This situation hampered practical use of EVs for drug delivery.
Foods are expected as alternative sources for the mass production of EVs. Previously, grapefruit-derived nanovesicles have been shown to be a cost-effective source of EVs for drug delivery [Citation10]. Since food is a widely available and inexpensive raw material for the production of EVs, food-derived EVs can be the ideal platform for the clinical application of EVs. Currently, bovine milk-derived EVs (mEVs) have emerged as a novel class of DDS [Citation11]. Similar to other body fluids, breast milk contains large amounts of EVs. First, mEVs have been identified in human breast milk [Citation12]. Similarly, mEVs from bovine milk were isolated and characterized [Citation13]. Our group demonstrated that bovine mEV contains mRNA and miRNA [Citation14]; thus, mEVs may deliver these RNAs to recipient cells [Citation15].
According to the literature, mEVs have a biological function, such as an immunomodulatory effect [Citation13]. In addition, mEVs and their cargos can be absorbed upon oral administration in humans [Citation16]. Recently, mEVs have been used as vesicles for the delivery of anti-cancer drugs [Citation11,Citation17,Citation18]. These studies demonstrated the feasibility of mEVs for drug-delivery applications.
To date, various isolation methods of mEVs have been developed, including ultracentrifugation [Citation11,Citation13,Citation19] and size-exclusion chromatography [Citation20]. However, none of them is suitable for the scalable production of mEVs. These methods require laborious repeated centrifugation steps to remove non-EV proteins. Furthermore, these reports did not carefully evaluate the purity of mEVs. This situation led us to develop a new method to purify pure mEVs from bovine milk utilizing a simple procedure.
In this study, we observed that acid treatment can remove non-EV proteins from defatted milk. Subsequent ultracentrifugation can isolate and concentrate up to 13.6 µg of mEVs from 1 mL of whey. We characterized mEVs using various physicochemical and biochemical analyses. Cellular uptake and cytotoxicity of mEVs were evaluated in vitro. Furthermore, systemic toxicity and immunogenicity of mEVs were evaluated upon intravenous administration in mice.
Materials and methods
Isolation of mEVs
Acetic acid/ultracentrifugation (AA/UC) method
Defatted bovine milk was purchased from a local supermarket. Defatted milk was pre-warmed for 10 min at 37°C and then mixed with acetic acid [milk/acetic acid = 100 (vol.)] for 5 min at room temperature followed by centrifugation at 10,000 g for 10 min at 4°C. Supernatant was filtered with a 0.22-µm membrane and designated whey. The whey was ultracentrifuged at 210,000 g for 70 min at 4°C using an SW41Ti rotor and an Optima XE-90 Ultracentrifuge (Beckman Coulter, Brea, CA, USA). A pellet of mEVs was resuspended with phosphate-buffered saline (PBS) and ultracentrifuged again. After the wash, the pellet of mEVs was resuspended in PBS, and the residual precipitates were removed via centrifugation at 10,000 g for 5 min at 4°C.
Centrifugation/ultracentrifugation (C/UC) method
A conventional isolation method was performed according to the previous article [Citation11]. Whole bovine milk was purchased from a local supermarket. Whole milk was centrifuged at 13,000 g for 30 min. After the collection of the middle layer (whey), whey was ultracentrifuged at 100,000 g for 60 min at 4°C to remove larger particles. The supernatant, crude mEV fraction, was further ultracentrifuged at 130,000 g for 60 min at 4°C. The pellet was washed with PBS twice, and mEVs were resuspended in PBS.
Characterization of mEVs
Biochemical characterization of mevs
Protein concentration of mEVs was determined using the Qubit Protein Assay kit (Thermo Fisher, Waltham, MA, USA). For the detection of total protein in raw material and mEV fraction, the samples were separated using SDS-PAGE and stained with silver. The EV marker proteins of mEVs were detected via Western blotting using anti-CD81 (clone 12C4, Cosmo Bio, Tokyo, Japan), anti-Rab5B (sc-598, Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-TSG101 (A303-507A-T, Bethyl Laboratories, Montgomery, TX, USA) and anti-HSC70 (MABE1120, Merck Millipore, Darmstadt, Germany) antibodies.
Physicochemical characterization of mEVs
Particle sizes and concentrations of mEVs were measured via nanoparticle tracking analysis (NTA) using NanoSight LM10 (Malvern Instruments Ltd, Malvern, UK). The morphology of mEVs was observed using a JEM-1400 plus (JEOL Ltd, Tokyo, Japan) transmission electron microscope (TEM) equipped with Veleta 2K x 2K CCD camera (Olympus, Tokyo, Japan). For the TEM observation, EV samples were negatively stained with 2% uranyl acetate.
Proteome analysis of mEVs
For the proteome analysis, mEVs were precipitated with trichloroacetic acid, followed by reduction, alkylation with iodoacetamide and trypsinization. The sample was separated using HPLC [EASY-nLC 1200 (Thermo Fisher)] and analysed via mass spectrometry using Q Exactive Plus (Thermo Fisher). The output data were analysed using the Scaffold software version 4.8.4 (Proteome Software Inc., Portland, OR, USA).
Cell culture
Mouse macrophage cell line, Raw264.7 (purchased from ATCC, Manassas, VA, USA), was maintained in Dulbecco’s modified Eagle medium (DMEM) containing 10% fetal bovine serum at 37°C in a 5% CO2 atmosphere. For the cellular uptake assay, mEVs were labelled with PKH26 (Sigma-Aldrich, St. Louis, MO, USA) according to the previous report [Citation21]. Raw264.7 cells were seeded on a glass-bottomed eight-well chamber (5 × 104 cells/well). Before the cellular uptake assay, cells were washed once with PBS and cultured in serum-free Advanced DMEM (Thermo Fisher). Cells were contacted with 10 µg of PKH26-labelled mEVs for 3 h at 37°C or 4°C. After the incubation, the cells were washed twice with PBS, fixed with 4% paraformaldehyde, stained with ActinGreen 488 ReadyProbes (Thermo Fisher) and then mounted with a Vectashield mounting medium with DAPI (Vector, Burfingame, CA, USA). Cells were observed using a laser scanning microscope Fluoview FV10i (Olympus). For the evaluation of cytotoxicity of mEVs, the cells were seeded in a 96-well plate (2 × 104 cell/well) and contacted with up to 200 µg/mL of mEVs for 24 h at 37°C. After the incubation, cell viability was measured using the Cell Counting Kit-8 (Dojindo, Kumamoto, Japan) according to the manufacturer’s instructions.
Systemic toxicity and blood cytokine level in mice
The animal experiments performed in this study were approved by the National Cancer Center Institutional Animal Care and Use Committee (approved number, T16-012-MB02). Female 7-week-old ICR mice (Charles River Laboratories Japan, Inc., Kanagawa, Japan) received mEVs intravenously via the tail vein. Each experimental group contained three mice. After 3 h (single injection) or 14 days (four times, 2-day interval), blood was collected via cardiac puncture under isoflurane anaesthesia. Biochemical analysis of mouse blood was performed by KAC Co., Ltd, Kyoto, Japan. The haematological test was performed by Kotobiken Inc, Tokyo, Japan. The cytokine level in blood was measured by Luminex System using the MILLIPLEX MAP Mouse Cytokine/Chemokine Panel 1 Pre-mixed 32Plex (Merck Millipore) according to the manufacturer’s instructions.
Anaphylaxis test
An anaphylaxis test was performed according to the literature [Citation22]. This experiment was performed by KAC Co., Ltd. Female 3-week-old C3H/HeNCrl mice (Charles River Laboratories Japan, Inc.) were used in this experiment. Briefly, the mice were immunized by intravenous injection of mEVs via the tail vein (five times, weekly). Each experimental group contained five mice. For the systemic anaphylaxis experiment, immunized mice received 150 µg of mEVs intravenously twice with a 30-min interval. After the injection, the symptoms were observed for 60 min. The symptoms were scored from 0 (no symptoms) to 5 (death), as described previously [Citation22]. For the blood histamine level measurement, blood was collected from immunized mice 30 min after the challenge, and histamine concentration was measured using ELISA (Enzo Life Sciences, Plymouth Meeting, PA, USA). For the passive cutaneous anaphylaxis (PCA) assay, blood was collected from immunized mice 1 week after the last immunization, and the serum was prepared. The serum was intradermally injected to the flank of naive mice (two injection sites per mice). After 24 h, 100 µL of 0.5% Evans blue was intravenously injected. Simultaneously, 50 µg of mEVs was intradermally injected to the flank of mice. After 30 min, the mice were euthanized, the skin of the abdomen was inverted, and reactions were examined for blue colour. The PCA reaction was scored as positive if the bluing of the skin at the injection sites was larger than 3 mm in diameter.
Results
Characteristics of mEVs
Bovine milk is an extremely complex material for isolating EVs. Whole milk contains abundant non-EV proteins, sugars, milk fat and other components. For the isolation of pure mEVs, all non-EV components should be removed. We combined acid precipitation and ultracentrifugation to isolate mEVs ()). To avoid the cumbersome skimming process, we chose commercially available defatted milk with a fat content below 0.3% (whole milk contains over 3.5% of fat). Non-EV proteins were precipitated from defatted bovine milk using acetic acid. After the acid precipitation, proteins in defatted milk were significantly removed ()). The supernatant was filtered and ultracentrifuged to isolate mEVs. The yield of the mEV fraction was 13.6 ± 2.2 µg/mL-whey (). Compared with the previously reported method (C/UC method), our method (AA/UC method) yielded over 20 times less protein content, indicating that the AA/UC method efficiently eliminates non-mEV proteins. The mEV fraction isolated via the AA/UC method abundantly contains EV marker proteins, CD81, Rab5B, TSG101, and Hsc70, whereas the mEV fraction isolated via the C/UC method does not (). The mEVs isolated using both methods showed a homogenous size distribution with a median diameter size of 120 nm () and ). Using the TEM, mEVs isolated via the AA/UC method showed a spherical shape with a lipid bilayer, with an approximately 100-nm diameter (upper panel in )). In contrast, mEVs isolated via the C/UC method contained spherical vesicles as well as protein aggregates, probably casein micelles (lower panel in )). Thus, even after the purification steps, the mEV fraction isolated by C/UC method still contains non-EV proteins.
Table 1. Purification of milk-derived EVs.
Figure 1. Isolation and characterization of mEVs from defatted bovine milk. (a and b) Workflow of isolation of mEV via the AA/UC (a) and C/UC (b) methods. (c) Silver staining (upper panel) and Western blotting (lower panel) of mEVs and their raw materials. Five micrograms per lane of mEVs was loaded. Five microlitres per lane of other samples was loaded. (d) Western blotting of mEVs isolated via the AA/UC and C/UC methods. (e) NTA of mEVs isolated via the AA/UC method. (f) Transmission electron microscopy observation of mEVs isolated via the AA/UC or C/UC method. (g) Venn diagram of detected protein numbers by proteome analysis in mEVs isolated by AA/UC or C/UC method.
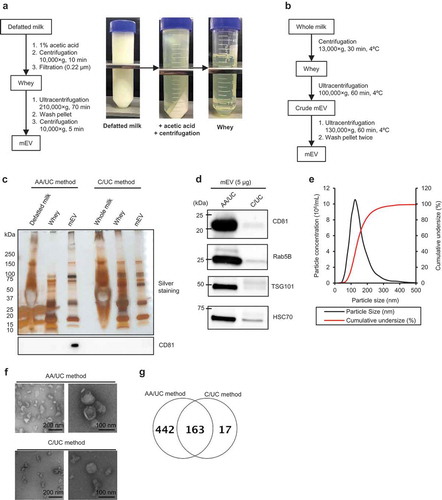
According to the proteome analysis, a total of 605 proteins were detected in the mEV fraction from AA/UC method () and Supplementary ). Among them, typical EV marker proteins, including tetraspanins, Rab proteins and others were detected, while whey proteins were still detectable (). Compared with the AA/UC method, the C/UC method enriched only 180 proteins ()). We were able to isolate mEV from defatted bovine milk.
Table 2. Summary of proteome analysis of mEVs.
Cellular uptake and cytotoxicity of mEVs
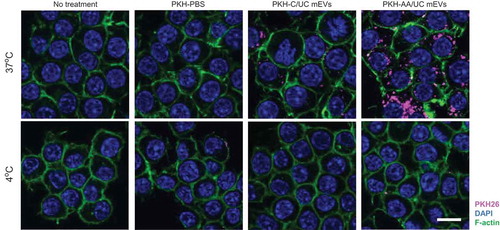
To confirm whether the mEVs isolated using our method can be used for the DDS, we performed a cellular uptake assay. After labelling with a hydrophobic fluorescence dye PKH26, mEVs purified by different methods were incubated with Raw264.7 cells. After 3 h at 37°C, abundant fluorescence signal was observed in the cells treated with PKH-labelled mEVs, while the negative control (PKH-PBS) showed no fluorescence signal (). Compared with mEVs prepared by the C/UC method, mEVs isolated from AA/UC seem to be taken up more efficiently by cells. Furthermore, cell incubation with mEVs at 4°C did not incorporate mEVs, suggesting that mEVs are taken up by an energy-dependent process, probably endocytosis.
Figure 2. Cellular uptake of PKH26-labelled mEVs. Raw264.7 cells were contacted with PKH26-labelled mEVs (prepared by C/UC or AA/UC method) for 3 h at 37 or 4°C and observed under a confocal microscope. As a negative control, the cells were left untreated or PKH26-labelling solution without mEVs. Bar indicates 10 µm.
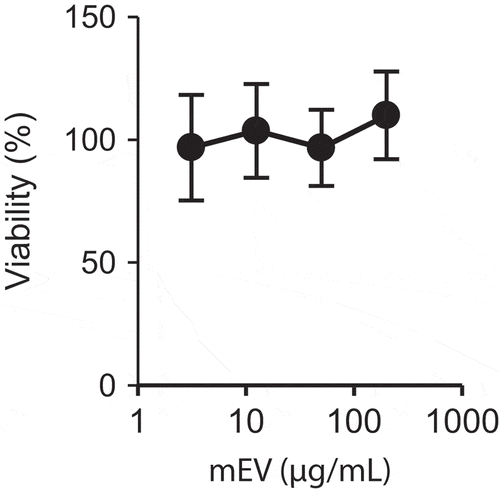
Cytotoxicity of mEV was measured using the Raw264.7 cells. After the incubation with mEVs, the viability of cells remained nearly 100%, even at the highest concentration (). From this result, we considered that mEVs have no cytotoxicity in vitro.
Toxicity of mEVs in mice
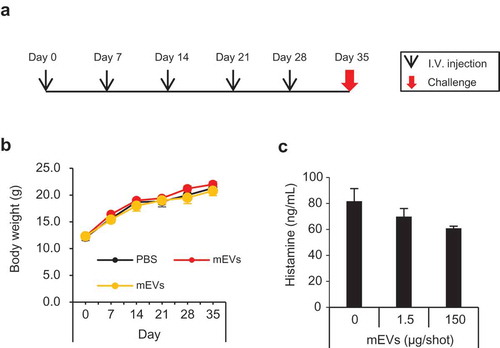
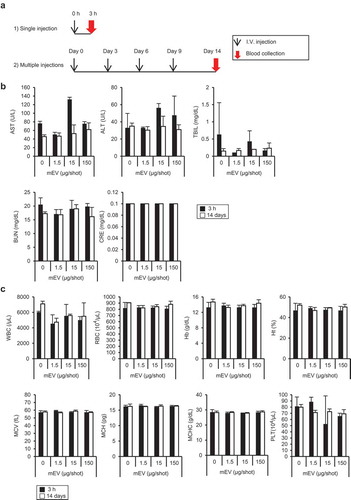
We evaluated the toxicity of mEV in ICR mice ()). After the intravenous injection of mEVs, systemic toxicity was not observed in all groups. Injection of mEV had no effect on the liver and kidney toxicity markers regardless of the regimens ()). Similarly, haematological parameters remained at the normal level after the injection of mEVs ()). Furthermore, the blood level of major inflammation cytokine, such as interleukin-12 (IL-12) and interferon gamma (IFN-γ), remained at the normal level after the systemic injection of mEVs. Although some types of cytokines in blood, such as IL-6 and granulocyte-colony stimulating factor (G-CSF), were increased 3 h after the single injection, cytokine levels became normal after 14 days with four injections (Supplementary Data). Moreover, major inflammatory cytokines, such as interferon gamma and IL-12, were at nearly undetectable levels after the injection of mEVs. According to these results, we considered that mEVs are well tolerated in mice upon systemic administration in mice.
Figure 4. Evaluation of systemic toxicity of mEVs in ICR mice. (a) Experimental design of administration of mEV into mice. (b) Blood level of liver-damage markers (AST, ALT, and TBIL) and kidney-damage markers (BUN and CRE) and (c) haematological parameters upon administration of mEVs. Black and white bars indicate 3 h and 14 days after administration, respectively. N = 3, mean ± standard deviation. ALT, Alanine transaminase; AST, aspartate transaminase; BUN, blood urea nitrogen; CRE, creatinine; TBIL, total bilirubin.
Immunogenicity of mEVs
C3H/HeNCrl mice were used for the evaluation of anaphylaxis according to the previous report [Citation22]. The mice received mEVs five times weekly and challenged on day 35 ()). During the experiment, the body weight of mice gradually increased over time, and no difference was observed between the groups ()). After the challenge, no mice showed systemic anaphylaxis symptoms (). The histamine level in blood was not elevated after the challenge ()). Furthermore, PCA tests revealed that blood in immunized mice did not contain anaphylaxis-inducing immunoglobulins (). Thus, it was strongly suggested that mEVs did not induce anaphylaxis in mice after serial intravenous injection.
Table 3. Systemic anaphylaxis test.
Table 4. Passive cutaneous anaphylaxis (PCA) test.
Discussion
In this study, we isolated mEVs from defatted milk by combining acid treatment and ultracentrifugation. Since majority of whey protein is casein (up to 80% of total whey protein), removal of casein is the key for obtaining pure mEV fraction. Although previous reports employed centrifugation to remove casein [Citation11], it is difficult to separate casein from mEVs, since colloidal characteristics of casein micelles are similar to mEVs (diameter and density are 100 nm [Citation23] and 1.08 g/mL [Citation24], respectively, for casein micelles). Acid precipitation is frequently used to remove casein from whole milk, owing to the low pI of casein (pH 4.5 [Citation25]). In addition, we confirmed that mEVs can be isolated from whole milk freshly collected from cow via the skimming process prior to the acid treatment (data not shown). The yield of mEV was over 10 µg/mL-whey (). Compared with the previously reported C/UC method [Citation11], our method yields 20-fold lower amounts of mEVs. Another report employing size-exclusion chromatography showed that the yield of mEVs from bovine milk is from 51 to 114 µg/mL [Citation20]. The relatively low protein yield of our method is probably due to the efficient elimination of non-EV protein via the acid precipitation. This is evidenced by our experiment in that the mEV fraction obtained via the AA/UC method is richer in EV marker proteins than that obtained via the C/UC method ()). Furthermore, the mEV fraction of the C/UC method contains fewer EV-like nanoparticles and much protein aggregates probably owing to the inefficient removal of casein micelles ()). The ratio of particle number per microgram of protein was approximately 2 × 109 (). This is consistent with the estimation that EVs have 109 particles/µg-protein [Citation26]. The low ratio of mEVs produced via the C/UC method (0.24 × 109 particles/µg-protein) may reflect the low purity. However, another paper stated that the particle/protein ratio of the purest EVs may be 2 × 1010 particles/µg-protein [Citation27], suggesting that our method can be further improved to isolate purer mEVs. Indeed, proteome analysis found that the mEV fraction contains non-EV proteins such as whey proteins and apolipoproteins (). Moreover, acid precipitation may remove not only non-EV proteins but also a part of mEVs. We assumed that the yields can be further improved by modifying the method in a future study. Furthermore, the yield of mEVs was far beyond that of cell-culture-derived mEVs. In our experiments, the yield of mesenchymal stem cell-derived EVs was between 0.1 and 0.3 µg/mL-conditioned medium (data not shown). Compared with this result, our method based on the bovine milk was 30–100-fold more efficient than the conditioned medium for collecting EVs. In addition to the yield, the cost of raw material is critical for the practical application. Based on our calculation of cost, bovine milk is at least 1000-fold more cost-effective than the serum-free medium for the production of the same amounts of EVs. Thus, the production of mEVs from bovine milk is advantageous for the practical application of EVs, including DDS, compared with the conditioned medium.
The proteome analysis in this study ( and Supplementary ) correlated well with a previous report showing that mEVs from bovine milk contain tetraspanins, heat shock proteins and other EV markers [Citation28]. However, we could not detect some of the EV proteins, such as MFG-E8 (lactadherin), while this protein is frequently found in the mEV fraction [Citation13,Citation20]. This is probably due to the differences in the isolation method, such as acid treatment and ultracentrifugation processes. Importantly, we observed that the mEV fraction of the C/UC method contains abundant whey proteins such as casein, albumin, lactoferrin, and lactoglobulin (Supplementary ). In the mEVs isolated by the C/UC method, these four non-EV proteins comprised 27.7% of the total spectrum detected in the proteome analysis, leading to less protein detection, whereas less than 4% were occupied by four whey proteins in mEVs isolated by the AA/UC method. From this result, we considered that the AA/UC method can isolate purer mEV than the C/UC method.
It was found that mEVs are readily taken up by the Raw264.7 cells (). In the previous report, mEVs were internalized into human macrophage cells [Citation15]. Another report demonstrated that mEVs from bovine milk deliver biologically functional miRNA into human cells and affect gene expression of recipient cells [Citation16]. From these results, mEVs are useful DDS for the delivery of nucleic acid medicine, such as miRNA and siRNA. In addition, mEVs were found to be internalized by endocytosis, since a low temperature significantly reduced the uptake of mEVs. Our results suggest that mEVs isolated using our method can deliver cargo molecules intracellularly. Moreover, even at higher concentrations, mEVs showed no cytotoxicity (). These results support the conclusion that the mEV is a useful and safe DDS.
In the in vivo experiments (), mEVs did not show any systemic toxicity at the highest dose (150 µg/shot), which corresponds to the 6 mg/kg (based on the estimation that body weight of female 7-week-old ICR mice is approximately 25 g). Furthermore, inflammatory cytokines were not elevated upon administration of mEVs (Supplementary Data). Thus, we considered that mEVs are well tolerated and non-immunogenic in mice. Since the safety was approved in mice, the mEVs are probably applicable for the DDS in human. In addition to systemic toxicity, anaphylaxis was not observed in immunized mice ( and and ). These results indicated that intravenously injected mEVs do not elicit anaphylaxis-inducing immunoglobulin E. However, we should mention that milk is one of the major causes of food allergy. Up to 0.1% of adults suffer from milk allergies [Citation29], while infants have milk allergies more frequently [Citation30]. These populations cannot receive mEV-containing formulation.
While we focused on intravenous administration of mEVs in this study, oral administration is an alternative route for drug delivery utilizing mEVs. As described previously, gavage of bovine milk did not induce anaphylaxis in mice [Citation22], showing that mEVs are non-immunogenic via oral administration. It was suggested that orally administered mEVs and their cargo RNA can be adsorbed and go into the blood circulation of mice [Citation16]. Furthermore, oral administration of mEV encapsulating anti-tumour agents can suppress tumour growth in the mouse xenograft model [Citation11,Citation18]. Taken together, orally and intravenously administered mEVs can deliver therapeutic molecules. mEVs may be a biocompatible material with non-immunogenic properties in humans.
Conclusions
We established the isolation method of mEVs. Furthermore, mEVs showed no systemic toxicity and immunogenicity in mice. We speculated that isolated mEVs can be used for various applications. Human-milk-derived mEVs have been shown to modulate the functionality of immune cells [Citation12,Citation31]. Similar to human mEVs, bovine mEVs may have a biological function, as described previously [Citation16]. It is possible that mEVs from bovine milk can be used as an immunomodulatory agent. Cosmetics and food additives are another attractive application of mEVs. Our method may enable broad application of mEVs.
Supplementary_data_Yoshioka_.zip
Download Zip (300.7 KB)Acknowledgements
We thank Prof. Iwano at Rakuno Gakuen University and Dr Muroya at National Agriculture and Food Research Organization for their helpful comments. The technical assistance of Tomomi Imamura was greatly appreciated. We also appreciated excellent assistance of Hiroko Omori of Core Instrumentation Facility at Research Institute for Microbial Diseases at Osaka University, for TEM observation.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplemental data
Supplemental data can be accessed here.
Additional information
Funding
References
- Yáñez-Mó M, Siljander PR-M, Andreu Z, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4:1.
- Ratajczak J, Miekus K, Kucia M, et al. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia. 2006;20(5):847–10.
- Valadi H, Ekström K, Bossios A, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–659.
- EL Andaloussi S, Lakhal S, Mäger I, et al. Exosomes for targeted siRNA delivery across biological barriers. Adv Drug Deliv Rev. 2013;65(3):391–397.
- Somiya M, Yoshioka Y, Ochiya T. Drug delivery application of extracellular vesicles; insight into production, drug loading, targeting, and pharmacokinetics. AIMS Bioeng. 2017;4(1):73–92.
- Tominaga N, Yoshioka Y, Ochiya T. A novel platform for cancer therapy using extracellular vesicles. Adv Drug Deliv Rev. 2015;95:50–55.
- Alvarez-Erviti L, Seow Y, Yin H, et al. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29(4):341–345.
- Ohno S, Takanashi M, Sudo K, et al. Systemically injected exosomes targeted to EGFR deliver antitumor MicroRNA to breast cancer cells. Mol Ther. 2012;21(1):185–191.
- Watson DC, Bayik D, Srivatsan A, et al. Efficient production and enhanced tumor delivery of engineered extracellular vesicles. Biomaterials. 2016;105:195–205.
- Wang Q, Zhuang X, Mu J, et al. Delivery of therapeutic agents by nanoparticles made of grapefruit-derived lipids. Nat Commun. 2013;4(May):1867.
- Munagala R, Aqil F, Jeyabalan J, et al. Bovine milk-derived exosomes for drug delivery. Cancer Lett. 2016;371(1):48–61.
- Admyre C, Johansson SM, Qazi KR, et al. Exosomes with immune modulatory features are present in human breast milk. J Immunol. 2007;179:1969–1978.
- Hata T, Murakami K, Nakatani H, et al. Isolation of bovine milk-derived microvesicles carrying mRNAs and microRNAs. Biochem Biophys Res Commun. 2010;396(2):528–533.
- Izumi H, Kosaka N, Shimizu T, et al. Bovine milk contains microRNA and messenger RNA that are stable under degradative conditions. J Dairy Sci. 2012;95(9):4831–4841.
- Izumi H, Tsuda M, Sato Y, et al. Bovine milk exosomes contain microRNA and mRNA and are taken up by human macrophages. J Dairy Sci. 2015;98(5):2920–2933.
- Baier SR, Nguyen C, Xie F, et al. MicroRNAs are absorbed in biologically meaningful amounts from nutritionally relevant doses of cow milk and affect gene expression in peripheral blood mononuclear cells, HEK-293 kidney cell cultures, and mouse livers. J Nutr. 2014;144(10):1495–1500.
- Munagala R, Aqil F, Jeyabalan J, et al. Exosomal formulation of anthocyanidins against multiple cancer types. Cancer Lett. 2017:1–9. DOI:10.1016/j.canlet.2017.02.004
- Agrawal AK, Aqil F, Jeyabalan J, et al. Milk-derived exosomes for oral delivery of paclitaxel. Nanomed Nanotechnol Biol Med. 2017. DOI:10.1016/j.nano.2017.03.001
- Zonneveld MI, Brisson AR, van Herwijnen MJC, et al. Recovery of extracellular vesicles from human breast milk is influenced by sample collection and vesicle isolation procedures. J Extracell Vesicles. 2014;3:1–12.
- Blans K, Hansen MS, Sørensen LV, et al. Pellet-free isolation of human and bovine milk extracellular vesicles by size-exclusion chromatography. J Extracell Vesicles. 2017;6(1):1294340.
- Takahashi Y, Nishikawa M, Shinotsuka H, et al. Visualization and in vivo tracking of the exosomes of murine melanoma B16-BL6 cells in mice after intravenous injection. J Biotechnol. 2013;165(2):77–84.
- Li X, Schofield BH, Huang C-K, et al. A murine model of IgE-mediated cow’s milk hypersensitivity. J Allergy Clin Immunol. 1999;103(2):206–214.
- Walstra P. Casein sub-micelles: do they exist? Int Dairy J. 1999;9(3–6):189–192.
- Bouchoux A, Ventureira J, Gésan-Guiziou G, et al. Structural heterogeneity of milk casein micelles: a SANS contrast variation study. Soft Matter. 2015;11(2):389–399.
- Chaiyasut C, Tsuda T. Isoelectric points estimation of proteins by electroosmotic flow: pH relationship using physically adsorbed proteins on silica gel. Chromatogr Chromatogr Sci. 2001;22(2):91–96.
- Sverdlov ED. Amedeo Avogadro’s cry: what is 1 μg of exosomes? BioEssays. 2012;34(10):873–875. DOI:10.1002/bies.201200045
- Webber J, Clayton A. How pure are your vesicles? J Extracell Vesicles. 2013;2(7):1–6.
- Reinhardt TA, Lippolis JD, Nonnecke BJ, et al. Bovine milk exosome proteome. J Proteomics. 2012;75(5):1486–1492.
- Woods RK, Thien F, Raven J, et al. Prevalence of food allergies in young adults and their relationship to asthma, nasal allergies, and eczema. Ann Allergy Asthma Immunol. 2002;88(2):183–189.
- Rona RJ, Keil T, Summers C, et al. The prevalence of food allergy: A meta-analysis. J Allergy Clin Immunol. 2007;120(3):638–646.
- Kosaka N, Izumi H, Sekine K, et al. microRNA as a new immune-regulatory agent in breast milk. Silence. 2010;1(1):7.