ABSTRACT
The abstracts presented at the 2018 International Society for Extracellular Vesicles Annual Meeting offer unique insight into the newest discoveries related to the biology and applied use of extracellular vesicles (EVs). As an extension of a recent “Clinical-Wrap Up” discussion at the International Society for Extracellular Vesicles 2018 Annual Meeting, a systematic review of each abstract was performed to determine which abstracts could be considered clinical research. Once the clinical research abstracts were identified, systematic data extraction included: the major focus of each clinical research abstract; the countries in which the work was done; and the sample size, if provided in the abstract. Each abstract was reviewed by two independent authors, with a third author resolving discrepancies in cases of disagreement. 174 out of 656 (27%) unique abstracts were determined to be clinical research. Oncology was a principal research focus (51 of the 174 clinical research abstracts, 29%). Many other clinical research abstracts presented at the International Society for Extracellular Vesicles 2018 Annual Meeting focused on the use of human samples for development of methods for potential application in the clinic. Beyond oncology and methods development, a wide range of topics was represented, including cardiovascular disease, neurodegenerative disease, genetics, and many others. Current research involving EVs highlights the common, but false dichotomy of science into curiosity-driven basic science or application-driven clinical research, when in fact both quest for understanding and intent to apply the findings appeared to drive much of the work at the International Society for Extracellular Vesicles 2018 Annual Meeting. Using Pasteur’s Quadrant as a framework, we discuss where the field of EV research is heading and how we may gain insight into the biological function of EVs in tandem with how they may benefit individual health.
Introduction
In his book “Pasteur’s Quadrant,” Donald Stokes wrote eloquently about the movement of new ideas from the realm of basic science to large-scale application [Citation1]. He proposed a model contrasting with what he perceived to be the dominant understanding: that ideas proceed from curiosity-driven basic science to applied research to a development cycle to production and operations. Rather than dividing research into basic research driven only by curiosity and applied research concerned only with effectiveness, he identified a third, hybrid motivation for some programmes of research. Stokes described “use-inspired basic science” as a type of research motivated simultaneously by curiosity and an intent to apply the results to a real-world problem. He considered Pasteur an exemplar of this important approach to research.
The commonly used definitions of clinical research highlight the false dichotomy of biomedical research into applied (clinical) research that generates no new fundamental insights into biology but improves patients’ health and basic science pursued without regard for application. The commonly used definitions of clinical research do not require that study participants have a health problem or that the results of the research will change the care of patients. Moreover, the common definitions are broad enough to include research with a principal motivation to directly improve human health, to aid in the development of new technologies that might someday be useful in the clinic, or simply to understand mechanisms of disease. In this sense, differentiating between clinical and basic research abstracts is not a simple matter of distinguishing whether the scientists involved are concerned or unconcerned with the application of their findings. This manuscript arose as an extension of one of the authors’ (JBB) comments at the Clinical Wrap-up of the 2018 International Society for Extracellular Vesicles Annual Meeting. We recapitulate the major ideas of that presentation, explaining the types of clinical research presented at the International Society for Extracellular Vesicles 2018 Annual Meeting, including the major focus of the projects; the country or countries in which the work was done; and the sample size, if it was provided. We also propose “Pasteur’s Quadrant” as an important framework for understanding our emerging field, beyond the traditional dichotomy of applied versus basic science.
Methods
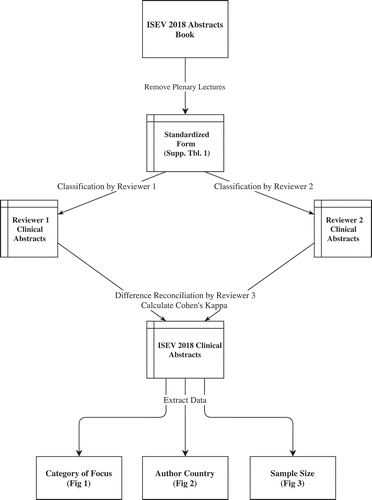
The International Society for Extracellular Vesicles 2018 Annual Meeting abstracts book [Citation2] served as the data source for this project. To ensure robust data extraction and to evaluate for ambiguity in the classification of clinical research, two authors (AZ and JBB) created independent extracts of the data. The analysis workflow is summarized in . First, using a standardized form (Supplemental Table), both of these authors independently examined every abstract in the International Society for Extracellular Vesicles 2018 Annual Meeting programme book to identify studies that should be considered clinical research. For this purpose, the authors were guided by the concept that clinical research involves human participants, whether through intervention or materials obtained from interaction with human participants [Citation3]. To maintain a focus on the new original science accepted for presentation at the meeting, the plenary lectures were not included in the analysis. To measure the agreement between the two authors regarding whether an abstract was clinical research, we calculated a metric called Cohen’s kappa, which accounts for chance agreement. After identifying the clinical research abstracts, the same two authors extracted: 1) the country or countries in which the work was done; 2) the major focus of the work; and 3) the sample size, if provided in the abstract. Abstracts with researchers from more than one country were tabulated under each country. An independent third reviewer reconciled any differences. Analysis was performed using the R statistical software, version 3.4.3 [Citation4]. To investigate the number of ongoing or completed clinical trials involving EVs, two searches of the main United States of American (USA) clinical trials registration website (clinicaltrials.gov) were conducted. The first search was for the term “exosomes.” The second search was also for the term “exosomes,” but results were limited to “interventional” studies that had results reported in clinicaltrials.gov. Finally, as a means of highlighting the breadth of topics of abstracts deemed clinical, five abstracts were selected at random for discussion from among those abstracts adjudicated as clinical.
Results
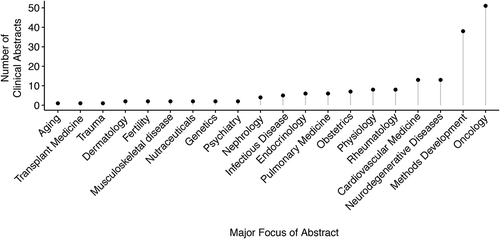
The reviewers informally noted that classifying the International Society for Extracellular Vesicles 2018 Annual Meeting work as clinical research was ambiguous in some cases. For example, in some instances, it was unclear whether the authors obtained clinical samples in a way that would allow the authors to know the identity of the study participant, an aspect of the National Institutes of Health (NIH) definition of clinical research. The reviewers occasionally came to different conclusions about whether the work was clinical research (kappa = 0.85, P < 0.001). After adjudication of these differences, 174 of 656 (27%) abstracts were determined to be clinical research. The clinical research abstracts presented at the International Society for Extracellular Vesicles 2018 Annual Meeting focused on a wide range of diseases and applications. These abstracts were placed in 20 categories of major focus () organized predominantly around clinical specialties (e.g., cardiovascular medicine), but also including methods development. While many categories of clinical research had fewer than 5 unique abstracts, oncology was a major focus, comprising 51 of the 174 clinical abstracts (29%). Of the 174 clinical abstracts, methods development (22%), cardiovascular medicine (7%), and neurodegenerative disorders (7%) were also commonly studied topics. Many studies investigated the fundamental biological effects of vesicles derived from a human biofluid. Within the major categories (e.g., cardiovascular medicine), specific diseases often constituted the major focus of clinical research abstracts at the International Society for Extracellular Vesicles 2018 Annual Meeting. Prostate cancer was the focus of 10 of the 50 cancer-related clinical research abstracts, whereas many different cancers were the topic of a single abstract.
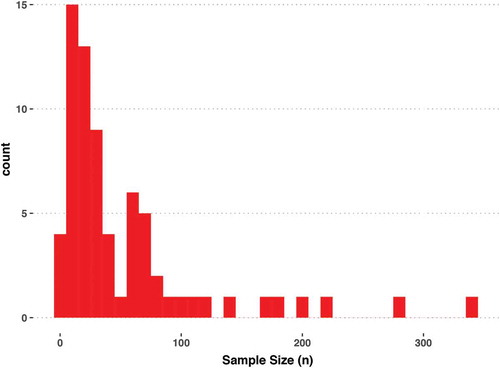
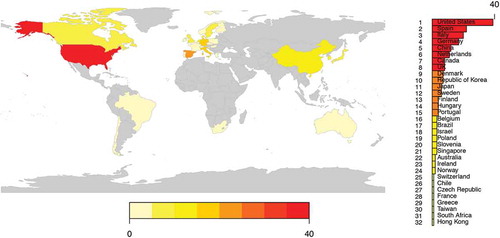
We next extracted features of the clinical research abstracts. Authors with affiliations in Australia, Europe, Africa, Asia, South America, and North America () were represented among the clinical research abstracts. Researchers in the USA served as authors for nearly 40 clinical research abstracts presented at the International Society for Extracellular Vesicles 2018 Annual Meeting. In descending order of author frequency, authors from Spain, Italy, Germany, China, the Netherlands, Canada, the United Kingdom, and 24 additional countries contributed to abstracts, as well (). Approximately 40% of the clinical abstracts (70 of 174) included the sample size. For most of the International Society for Extracellular Vesicles 2018 Annual Meeting clinical research abstracts for which a sample size was provided in the abstract, the number of study participants was fewer than 100 ().
Figure 3. The number of clinical abstracts from various countries presented at the 2018 International Society for Extracellular Vesicles Annual Meeting.
To place our findings in a larger context, we searched clinicaltrials.gov. A search for “exosomes” yielded 88 search results. However, the same search, when restricted to interventional studies with results in clinicaltrials.gov, returned 0 results.
The five randomly selected clinical abstracts reflect the diversity of the more clinically focused work presented at this meeting. Abstract PS07.03 focused on exosomes from both cultured malignant peripheral nerve sheath tumour cells and the plasma of patients with neurofibromatosis, highlighting the mix of more basic and more applied work so common at the International Society for Extracellular Vesicles 2018 Annual Meeting. PS06.06 described plasma EVs isolated from study participants during low or high particular matter days to study the influence of air pollution. Abstract PF01.14 reports results of an investigation of extracellular vesicle-associated deoxyribonucleic acid (DNA) and cell-free DNA in patients with metastatic melanoma. LBT01.04 reports a method of making good manufacturing practice-compliant clinical-grade exosomes. The final randomly selected clinical abstract was PF01.18, reporting the use of antibody- and peptide-based enrichment of tumour EVs from the plasma of patients with prostate cancer, as well as extraction and analysis of ribonucleic acid (RNA) and DNA.
Discussion
A striking aspect of the clinical research presented at the International Society for Extracellular Vesicles 2018 Annual Meeting is how many of the abstracts challenge traditional notions of applied versus basic research. We found many examples of projects involving the separation of human EVs to determine their properties when applied to cells in vitro. Although conducting work of this type requires an understanding of the regulatory environment governing human participants’ research, the intent of many of these studies appeared to be to generate a revised understanding of biology, rather than to directly improve a patient-related outcome. This is indeed basic science, even if it is also clinical research according to the NIH definition. Why are human samples so often the starting point of this work? Although the volume of sample available is greater than from a mouse or rat, we suspect a principal reason is because studying human biofluids is perceived as increasing the likelihood that the findings will someday help patients in the clinic. Many of these studies fall squarely into Stokes’ third category of research, rather than pure basic or pure applied research.
Researchers’ country of origin is unimportant in contrast to what they contribute to the world. On the other hand, countries’ funding and regulatory environment might affect clinical research output. For that reason, our analysis included mapping of authors’ countries. The International Society for Extracellular Vesicles 2018 Annual Meeting was truly an international meeting, with researchers from all continents (except Antarctica) presenting work. The preponderance of abstracts from the USA is arguably unsurprising in view of its large size relative to most other countries. It is encouraging to see that the meeting location remote from the USA did not prevent US researchers from contributing their work.
Does the lower number of study participants described in the International Society for Extracellular Vesicles 2018 Annual Meeting clinical research abstracts impugn the research present at the meeting? By no means. In the much older and more mature field of cardiovascular medicine, effective solutions to clinical problems often exist, requiring sponsors to fund large studies to demonstrate incremental improvements beyond the current standard of care. A use-inspired basic research project intended to determine whether DNA is present in exosomes in human plasma, in contrast, does not require thousands or even hundreds of people. Initial proof of concept might be provided by a careful n of 1 study with well-designed controls, in fact. Moreover, a subset of the clinical studies presented at the International Society for Extracellular Vesicles 2018 Annual Meeting involved serial measurement of analytes within individuals, using samples that were collected with special considerations for these emerging forms of clinical chemistry. This type of work involves different challenges compared to studies with a single point of contact with the patient, and the resources required for large-scale longitudinal participant and follow-up in clinical trials might not be available to investigators doing principally bench-based investigation. Moreover, within-subjects designs increase the power of such studies by allowing participants to serve as their own controls, minimizing noise. Add to these considerations the greater complexity of bespoke sample collection methods that facilitate high-quality research on EVs. Finally, even if a team finds the funds to scale up sample collection, scaling up vesicle isolation remains a technical challenge. Thus, the sample sizes in these early clinical studies are neither surprising, nor disappointing, but a reflection of the early state of the field. In the final analysis, the sample sizes reflect restraint on the part of funding agencies and investigators in pushing forward into the unknown. How long this degree of restraint will remain appropriate will be determined by how rapidly our field develops, which in turn depends upon factors such as sharing data and protocols.
Most widely read medical journals will not publish clinical trials that have not been registered, making registries like clinicaltrials.gov a window on completed and in-progress clinical trials. Beyond interventional clinical trials, investigators can register observational clinical studies. The finding of 0 registered interventional clinical trials with results in clinicaltrials.gov does not mean that no clinical trials with an intervention have been done. For example, once investigators publish their work, they will not necessarily return to clinicaltrials.gov to report results there, as well. Indeed, an example of a completed clinical trial can be found in the phase II clinical trial conducted by Besse et al. [Citation5]. Building on the group’s two prior phase I trials, this clinical trial evaluated the efficacy of injected dendritic cell-derived exosomes in enhancing natural killer cell immune responses in patients with advanced non-small cell lung cancer. The primary endpoint of at least 50% of patients with progression-free survival at 4 months after cessation of chemotherapy was not met. Nonetheless, this result does not diminish the importance of these efforts to improve outcomes in patients with this deadly disease, for as William Withering recognized in 1785, “…the knowledge of what will not do, may sometimes assist us to discover what will” [Citation6]. While this study was registered with clinicaltrials.gov, the authors chose publication as the venue for disseminating the results, rather than posting them to clinicaltrials.gov. Still, the complete lack of interventional clinical trials with associated results in clinicaltrials.gov suggests that relatively few such studies have been completed. Two types of extracellular vesicle-related clinical trials would be of greatest significance to clinical medicine: “strategy trials” in which patients are randomized to extracellular vesicle assay-guided treatment in a randomized trial or the current standard of care, and trials involving the administration of EVs. Strategy trials have historically been challenging, in part because of a strong inclination on the part of participating clinicians to continue the current standard of care, rather than to randomize patients. Whether strategy trials involving EVs or trials involving administration of EVs will become common remain to be seen.
Some limitations of our analysis bear mention. We could not be sure in many instances whether the authors had met the NIH definition of clinical research, which requires that samples be in some way traceable to the study participant. Whether or not this aspect of the NIH definition is met is less relevant to the spirit of this analysis compared to whether or not clinical samples were used to push forward the state of the art in extracellular vesicle research. In addition, many abstracts did not provide the sample size. It is possible that studies of larger sample size were included in the abstract book but did not mention the sample size in the abstract. We are aware of one such instance, the Extracellular RNA Consortium, which mentioned the number of clinical samples, but not the number of participants from which they were obtained. Although the Extracellular RNA Consortium has a large sample size that we were able to locate elsewhere, we suspect it is unlikely that most of the other abstracts with unspecified sample size are large. Finally, our clinicaltrials.gov search term “exosomes” does not necessarily capture all clinical trials involving EVs, nor do we assume that all studies returned by that search involve exosomes as the term is understood in our field. However, the term has the benefit of being specific, so that it is unlikely to appear in a study’s entry if the study has no connection to EVs. For that reason, we consider the term informative for our survey of clinical research involving EVs.
What’s next for clinical research involving EVs? Many questions remain to be clarified (). The upcoming revision of the Minimal Information Standards for Extracellular Vesicle Research (MISEV 2018) should clarify issues relevant to standardizing extracellular vesicle extraction from biofluids. A key issue is how to make extracellular vesicle isolation sufficiently straightforward and robust for use in a clinical laboratory. One of the authors (JBB) recently visited his hospital’s clinical laboratory and became familiar with the methods used for urinalysis. The process has been made so simple and automated that it consists of a “dip” of a stick in a urine sample and the interpretation of a resulting colour change by a machine. Clinical laboratories seek methods requiring no more than trivial sample handling, with a high degree of automation and with little or no laboratory technician judgment. How our field can deliver such tests to the clinical laboratory will be interesting to observe over time. EVs are poised to aid in our understanding of pathology and to potentially guide interventions. In this process, we stand to learn about the biology of EVs in tandem with their therapeutic potential, fulfilling the desire of curiosity and applicability, as Pasteur did generations before.
Table 1. Questions to be answered towards the clinical relevance of extracellular vesicle research.
Supplemental Material
Download MS Excel (34 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary Material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Stokes DE. Pasteur’s Quadrant: basic science and technological innovation. Washington (DC): Brookings Institution Press; 1997.
- The International Society for Extracellular Vesicles. Annual meeting abstract book. J of Extracell Vesicles. 2018;7(sup1):1–7.
- Glossary | Research Involving Human Subjects [Internet]. Bethesda (MD): National Institutes of Health; 2017 [updated 2018 Mar 22; cited 2018 Mar 22; cited 2018 Mar 22; cited 2018 May 22; cited 2018 May 17]. Available from: https://humansubjects.nih.gov/glossary
- R Core Team. R: A language and environment for statistical computing [Digital download]. Version 3.4.3. Vienna (AT): R Foundation for Statistical Computing; 2017.
- Besse B, Charrier M, Lapierre V, et al. Dendritic cell-derived exosomes as maintenance immunotherapy after first line chemotherapy in NSCLC. Oncoimmunology. 2015;5(4):13. 10.1080.
- Withering W. An account of the foxglove, and some of its medical uses: with practical remarks on dropsy, and other diseases. London: Printed by M. Swinney for G.G.J. and J. Robinson; 1785.