ABSTRACT
The past decade has witnessed an exponential development in the field of extracellular vesicles. Sporadic observations have reached a critical level and the scientific community increasingly recognizes the potential biomedical significance of these subcellular structures present in all body fluids as significant components of the cellular secretome. The Educational Committee of the International Society for Extracellular Vesicles prepared two posters (“Basic aspects of extracellular vesicles” and “Clinical aspects of extracellular vesicles”) to provide essential pieces of information on extracellular vesicles at glance for anyone not familiar with the field.
Basic and clinical aspects of extracellular vesicles
Since 2010, the scientific and clinical interest in extracellular vesicles (EVs) is growing exponentially. EVs are small, lipid membrane enclosed subcellular structures carrying biomolecules which are released by cells into their environment [Citation1–Citation3]. The term “EVs” is an umbrella term for all types of vesicles, including “exosomes” and “microparticles” or “microvesicles” [Citation4]. This term is endorsed by the International Society on Extracellular Vesicles (ISEV; www.isev.org), a global scientific society founded in 2011), because at present no biochemical or (bio)physical distinction can be made between types of EVs generated by different biogenesis pathways [Citation2].
The two posters by ISEV on “Basic aspects of extracellular vesicles” and “Clinical aspects of extracellular vesicles” provide a brief introduction about EVs to students and (bio) medical healthcare professionals.
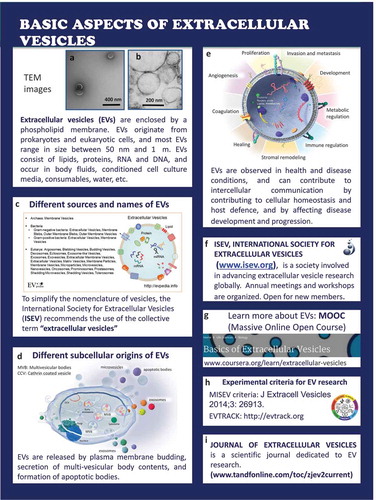
Basic aspects of extracellular vesicles ()
All cells release EVs into their environment, and (bio)fluids such as conditioned culture medium and body fluids contain EVs. When EVs are visualized by electron microscopy (A and B are EM microphotographs provided by Rienk Nieuwland and Agnes Kittel, respectively), EVs appear as small, usually spherical particles surrounded by a phospholipid bilayer membrane. This membrane often contains proteins from the parental cell, thus enabling the identification of the cell of origin. In addition, EVs contain biomolecules originating from the parental cells, including RNA and DNA, metabolites and lipids [Citation5].
Initially, EVs were often named after the cell type of origin, or after the organ in which they were discovered, such as dexosomes or prostasomes. Because this nomenclature was not only confusing but also resulted in development of parallel research fields lacking interaction, ISEV endorses the term “extracellular vesicles” as an umbrella term [C http://evpedia.info, Citation6].
Detailed analysis of the biochemical composition of EVs has shown that the overall composition of EVs differs from that of the releasing parental cells. This implies that cargo sorting mechanisms exits that affect the composition of EVs. Moreover, the biochemical composition depends on the “status” (resting, activated, etc.) of the parental cell. EVs can be released directly by budding from the plasma membrane, by secretion of prestored EVs in multi-vesicular bodies, by formation of apoptotic bodies as well as released from enveloped virus-infected cells (D). Due to all these variables, EVs are highly heterogeneous in size and composition [Citation7–Citation9].
EVs are not merely “innocent bystanders” but play important roles in physiology and pathology (E). In physiology, EVs contribute to homeostasis and promote host-defence mechanisms including haemostasis and inflammation [Citation3], whereas in pathology EVs may contribute to disease development and progression, for example in cancer [Citation10,Citation11]. Because EVs are capable of delivering their proteins, lipid and RNA cargo to target (recipient) cells, EVs can regulate gene expression and consequently the phenotype and biological functions of the target cell. Thus, by exchanging information between cells, EVs contribute to intercellular communication.
ISEV (F) developed a massive open online course on EVs that is freely available [Massive Online Open Course (MOOC) (G), http://coursera.org/learn/extracelllular-vesicles, Citation12], published minimal requirements for studies on EVs [MISEV, Citation13] (H). ISEV endorses a knowledge base that has been launched to monitor the quality of pre-analytical data reporting [http://evtrack.org, Citation14] (H). The official journal of ISEV is the Journal of Extracellular Vesicles (JEV) (I).
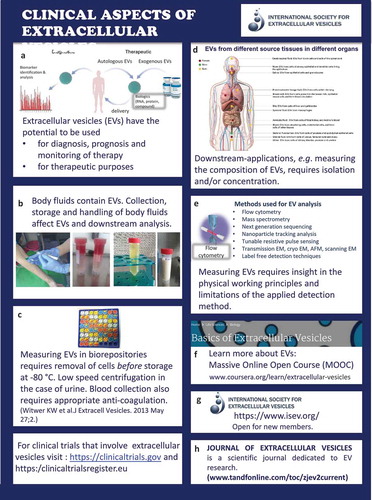
Clinical aspects of extracellular vesicles ()
In general, EVs have a huge potential for diagnosis of disease, prognosis and monitoring of therapy [Citation15]. Because EVs can be considered as naturally occurring autologous nanocarriers”, EVs can be loaded with drugs for local drug delivery (A).
At present, standard operation procedures are being developed for routine collection, storage and handling of biofluids and body fluids such as urine, blood and breast milk to enable the comparison of measurements between patients and controls and to allow exchange of data between institutes and instruments, etc. (B).
In most clinical studies, EV-containing biofluids will be collected in biorepositories. The pre-analytical conditions may differ between biofluids, but removal of cells and cell fragments is essential to circumvent contamination due fragmentation of cells during freeze thawing (C).
Because all human (and animal) body fluids contain EVs, and because the cellular origin, composition and functions of EVs change in disease, the detailed analysis of EVs is thought to behold clinically relevant information. To get access to this information, however, most downstream applications require either isolation and/or concentration of EVs (D).
Detection of (single) EVs and detailed insight into their biochemical composition by downstream methods such as proteomics requires a thorough understanding of the biochemical and (bio)physical limitations and possibilities of the various isolation, detection and characterization methods (E). In addition, the method of collection, handling and storage conditions of EV can alter the molecular composition of EV [Citation16,Citation17].
For more information, please visit the MOOC on EVs (F), the Journal of Extracellular Vesicles (JEV) (G) or contact the International Society for Extracellular Vesicles (H).
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Tkach M, Théry C. Communication by extracellular vesicles: where we are and where we need to go. Cell. 2016 Mar 10;164(6):1226–4.
- van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018 Apr;19(4):213–228.
- Yáñez-Mó M, Siljander PR, Andreu Z, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015 May;4(1):27066.
- György B, Szabó TG, Pásztói M, et al. Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell Mol Life Sci. 2011 Aug;68(16):2667–2688.
- van der Pol E, Böing AN, Harrison P, et al. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol Rev. 2012 Jul;64(3):676–705.
- Kim DK, Lee J, Kim SR, et al. EVpedia: a community web portal for extracellular vesicles research. Bioinformatics. 2015 Mar 15;31(6):933–939.
- Kowal J, Arras G, Colombo M, et al. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci U S A. 2016 Feb 23;113(8):E968–77.
- Soekmadji C, Riches JD, Russell PJ, et al. Modulation of paracrine signaling by CD9 positive small extracellular vesicles mediates cellular growth of androgen deprived prostate cancer. Australian prostate cancer collaboration bioresource. Oncotarget. 2017;8(32):52237–52255.
- Verweij FJ, Bebelman MP, Jimenez CR, et al. Quantifying exosome secretion from single cells reveals a modulatory role for GPCR signaling. J Cell Biol. 2018;217(3):1129–1142.
- Choi D, Lee TH, Spinelli C, et al. Extracellular vesicle communication pathways as regulatory targets of oncogenic transformation. Semin Cell Dev Biol. 2017 Jul;67:11–22.
- Sato S, Weaver AM. Extracellular vesicles: important collaborators in cancer progression. Essays Biochem. 2018 Apr 17;62:149–163.
- Lässer C, Théry C, Buzás EI, et al. The international society for extracellular vesicles launches the first massive open online course on extracellular vesicles. J Extracell Vesicles. 2016 Dec 16;5:34299.
- Lötvall J, Hill AF, Hochberg F, et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the international society for extracellular vesicles. J Extracell Vesicles. 2014;3:26913.
- Van deun J, Hendrix A. Is your article EV-TRACKed? EV-TRACK consortium. J Extracell Vesicles. 2017 Nov 10;6(1):1379835.
- Roy S, Hochberg FH, Jones PS. Extracellular vesicles: the growth as diagnostics and therapeutics; a survey. J Extracell Vesicles. 2018 Feb 26;7(1):1438720.
- Coumans FAW, Brisson AR, Buzas EI, et al. Methodological guideli120nes to study extracellular vesicles. Circ Res. 2017 May 11;120:1632–1648.
- Witwer KW, Soekmadji C, Hill AF, et al. Updating the MISEV minimal requirements for extracellular vesicle studies: building bridges to reproducibility. J Extracell Vesicles. 2017;6(1):1396823.