ABSTRACT
The oral mucosa exhibits unique regenerative properties, sometimes referred to as foetal-like wound healing. Researchers from our institute have used sheets of oral mucosa epithelial cells (OMECs) for regenerative medicine applications including cornea replacement and oesophageal epithelial regeneration for stricture prevention. Here, we have isolated exosomes from clinical-grade production of OMEC sheets from healthy human donors (n = 8), aiming to evaluate the clinical potential of the exosomes to stimulate epithelial regeneration and to improve understanding of the mode-of-action of the cells. Exosomes were isolated from conditioned (cExo) and non-conditioned (ncExo) media. Characterization was performed using Western blot for common exosomal-markers: CD9 and flotillin were positive while annexin V, EpCam and contaminating marker GRP94 were negative. Nanoparticle tracking analysis revealed a diameter of ~120 nm and transmission electron microscopy showed a corresponding size and spherical appearance. Human skin fibroblasts exposed to exosomes showed dose-dependent reduction of proliferation and a considerable increase of growth factor gene expression (HGF, VEGFA, FGF2 and CTGF). The results were similar for both groups, but with a trend towards a larger effect from cExo. To study adhesion, fluorescently labelled exosomes were topically applied to pig oesophageal wound-beds ex vivo and subsequently washed. Positive signal could be detected after as little as 1 min of adhesion, but increased adhesion time produced a stronger signal. Next, labelled exosomes were added to full-thickness skin wounds in rats and signal was detected up to 5 days after application. cExo significantly reduced the wound size at days 6 and 17. In conclusion, exosomes from OMEC sheets showed pro-regenerative effects on skin wound healing. This is the first time that the healing capacity of the oral mucosa is studied from an exosome perspective. These findings might lead to a combinational therapy of cell sheets and exosomes for future patients with early oesophageal cancer.
Introduction
The oral mucosa is a suitable cell source for tissue engineering and regenerative medicine applications, including cell sheet fabrication. It is easily accessible and exhibits unique characteristics such as foetal-like wound healing [Citation1] and antibacterial properties [Citation2,Citation3]. While skin wounds generally heal with scar (“reparation”), wounds in the oral mucosa are prone to heal without scar (“regeneration”) [Citation4]. Unfortunately, the underlying mechanism for the difference in healing is poorly understood. Being able to harness regenerative healing capabilities for use in other anatomical locations could be of great benefit for millions of people globally.
Stricture formation is a common complication to oesophageal surgery, which results in a decrease of the luminal diameter and can cause dysphagia [Citation5]. Oral mucosal epithelial cell (OMEC) sheets are currently used to improve mucosal healing and reduce the risk and severity of stricture after endoscopic removal of superficial tumours in the oesophagus. The method was first developed and investigated in a canine model, which revealed that the cell sheets enhanced wound healing and reduced inflammation in the ulcer site [Citation6]. Patients have since been treated in Japan [Citation7,Citation8] and in Sweden [Citation9]. The aim of this cell sheet therapy is to eliminate stricture formation, a complication that commonly requires multiple balloon dilatations to treat. Although the findings are promising some patients still develop strictures, particularly after large dissections [Citation9].
Exosomes are small vesicles which have been identified from a variety of cell sources. Increasing evidence suggests that a considerable portion of the therapeutic effect of cell therapy is attributed to soluble factors, including exosomes [Citation10,Citation11]. To this end, we investigated if conditioned media from the cell sheet production could be transformed into a therapeutic agent, possibly to use in combination with cell sheets in order to further reduce the risk of oesophageal strictures. This study also sheds light on the mode-of-action of the cell sheet therapy and on the unique regenerative capacity of the oral mucosa. During the cell sheet production, a large amount of conditioned media is produced. In the future, exosomes could potentially be isolated from this media and be used synergistically with the cell sheets.
Local injection of corticosteroids is another approach to prevent stricture formation which appears to be effective, but is associated with an increased risk of oesophageal perforation [Citation12]. Similar to scars [Citation13], oesophageal strictures [Citation14] are characterized by an abnormal increase in the production and deposition of collagen. Corticosteroids have also been used to prevent scar-formation in the skin [Citation15], with one mode-of-action being reduction of fibroblast proliferation, the major collagen-synthesizing cell [Citation16]. For these reasons, fibroblasts were selected as our target cell for in vitro experiments with dexamethasone as a control.
The oral cavity retains antibacterial properties as a part of the innate immunity. This defence mechanism is not entirely understood, but epithelial antimicrobial peptides, such as β-defensin, originating from the oral epithelium have been identified. These peptides operate by disrupting the microbe cell membrane [Citation17]. Progenitor cells from the oral mucosal lamina propria similarly secrete the antimicrobial peptides osteoprogetrin and haptoglobin, which, in effect, could reduce the growth of several pathogens [Citation2]. To this end, we evaluated the exosomes impact on growth of Staphylococcus aureus.
Media was collected from the production of clinical-grade cell sheets sourced from eight, healthy donors. Conditioned and non-conditioned media from each donor was processed separately (see study overview, ). The media included autologous serum meaning that the exosomes isolated from non-conditioned media were derived from the human serum, while exosomes from conditioned media consisted of a mix of serum exosomes and epithelial cell-exosomes. After isolation, the exosomes were used for characterization, in vitro, and in vivo studies.
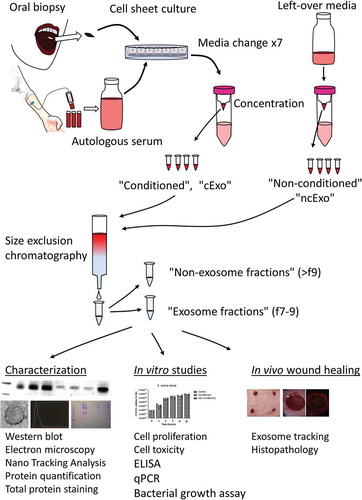
Figure 1. Study overview. Serum and oral mucosal tissue were collected from healthy donors and epithelial cells were isolated and cultured as cell sheets. The autologous serum was used in the culture media and exosomes isolated from this media represent an important control in downstream analyses (“non-conditioned”, “ncExo”). Exosomes were also isolated from used media that was collected from the cell cultures (“conditioned”, “cExo”). For exosome isolation, the media was concentrated and subjected to size exclusion chromatography. The exosomes were then used for characterization, in vitro, and in vivo studies.
Material and methods
Exosome isolation and characterization
Exosome isolation
Clinical-grade cell sheets were produced by CellSeed Inc. according to previously published methods [Citation7,Citation9]. Briefly, healthy donors’ oral cavity was sterilized with povidone-iodine and a biopsy was acquired from the buccal mucosa. Epithelial cells were isolated after dispase-treatment and seeded on temperature-responsive cell culture inserts (UpCell Insert; CellSeed Inc., Tokyo, Japan). The media (containing 5% autologous serum) was changed on days 5, 8, 10, 12, 13, 14 and 15. Conditioned media was also collected on the final day of culture (day 16). The media was stored in the fridge and processed within 3 days by centrifugation at 300 × g for 10 min at 4°C; afterwards the supernatant was filtered through a 0.22 µm syringe filter, concentrated using 100 kDa filters (Amicon Ultra-14, Merck Millipore, MA, USA) and stored in −80°C. The concentrated media was pooled and further concentrated using 10 kDa filter (Amicon Ultra-4, Merck Millipore) until the volume was less than 500 µL. Exosomes were isolated from bulk proteins using size exclusion chromatography (qEV, Izon) according to the manufacturer’s protocol. Fractions 7–9 were pooled as “exosome fractions” (“cExo”) and fractions 10–14 were collected separately as “non-exosome fractions”. Ten microlitres of fractions 7–9 (1.5 mL) were saved for total protein staining and the rest were concentrated using 10 kDa filter. Non-conditioned media was incubated for 48 h in 37°C with 5% CO2 before concentration with a 100 kDa filter and processed using a similar method as described above (“ncExo”). The samples were stored in −80°C until further use. These processes are summarized in .
Cell lysate preparation
Cell lysates were made from oral keratinocytes (HOK, ScienCell Research Laboratories, CA, USA). Cell suspensions (4 × 106 cells) were centrifuged at 300 × g for 5 min, the supernatant was discarded, and the pellet resuspended in PBS and centrifuged a second time. The pellet was resuspended in radioimmunoprecipitation assay buffer (RIPA, Sigma-Aldrich, MO, USA) with 1% protease/phosphatase inhibitor cocktail (Cell Signaling Technology, MA, USA) and sonicated for 5 min (15 s on, 15 s off intervals) in an ice bath. The sample was then centrifuged at 8000 × g for 10 min at 4°C and the supernatant was collected and aliquoted for downstream analyses.
Total protein concentration measurement
Protein concentrations of cell lysates, exosomes, and non-exosome-fractions were measured using Pierce™ BCA Protein Assay Kit (Thermo Fisher Scientific, Waltham, MA, USA). The samples were diluted 1:1 with RIPA and sonicated for 5 min (on/off: 15 s/15 s, in ice bath, high setting (Bioruptor, Cosmo Bio, Tokyo, Japan) and the BCA assay was performed according to manufacturer’s instructions.
Western blot
Three micrograms of cell lysate or exosome isolates were diluted 1:1 in Laemlli buffer (BioRad, CA, USA), heated to 95°C for 5 min and subsequently cooled on ice. The samples were loaded on NuPage 4–12% 1.5 × 15 wells gels (Thermo Fisher Scientific), subjected to electrophoresis (200 V, 125 mA, 25 min, iBlot Invitrogen), and proteins were then transferred to nitrocellulose membranes (Protein iBlot Gel Transfer stacks, Invitrogen) using iBlot (Invitrogen) 11-min programme. The following blocking antibody incubation and washing steps were performed on rocking platforms. The membrane was blocked using 5% dry milk (CellSignaling) in TBS-T (BioRad) for 60 min at room temperature and exposed to primary antibodies at 4°C, overnight. The membrane was washed 3 × 10 min in TBS-T on an orbital shaker and exposed to secondary antibody for 60 min at room temperature, followed by another 3 × 10 min TBS-T wash. The buffer was drained from the membrane and detection reagent (ECL Prime WB detection Kit, Sigma-Aldrich) was added for 5 min. The detection agent was drained and the signal was visualized using LAS 4000 mini (Fujifilm, Tokyo, Japan).
The antibodies and concentration used were:
CD9 (#13174, Cell Signaling Technologies, 1:1000, ref 02/2017, lot 1), Annexin 5 (#8555, Cell Signaling Technologies, 1:1000, ref 10/2016, lot 1), Flotillin (#18634, Cell Signaling Technologies, 1:1000, ref 10/2016 lot 1), GRP94 (#2104S, Cell Signaling Technologies, 1:1000, ref 04/2017, lot 2), HSP70 (#4876T, Cell Signaling Technologies, 1:1000, ref 10/2016, lot 2), EpCAM (#2626T, Cell Signaling Technologies, 1:1000, ref 10/2016, lot 1) and Anti-rabbit IgG, HRP-linked (#7074, Cell Signaling Technologies, 1:5000, ref 09/2016, lot 26).
The antibodies were diluted in “Can Get Signal” solution (Toyobo, Osaka, Japan). The membranes were stripped to reuse them with different antibodies; 30 min 2% SDS (Wako, Osaka, Japan), 0.8% beta-mercaptoethanol (Wako) Tris-HCl pH 6.6, at 50°C followed by 3 × 10 min TBS-T washes. Successful stripping was verified by adding detection reagent and visualization in LAS 400 mini (Fujifilm). The ladder used was Precision Plus Protein All Blue (BioRad).
Nanoparticle tracking analysis
Exosome size distribution and particle concentration was measured using NanoSight LM10 system (NanoSight Ltd., Salisbury, England), software version NTA2.3 using the following setting; detection level 7, camera level 14, detection time: 5 × 30 s.
Coomassie Brilliant Blue-staining of protein gels
To visualize protein content in the different fractions, protein electrophoresis with subsequent Coomassie Brilliant Blue (CBB) staining was performed. Ten microlitres of fraction 7–9, 10, 11, and 12 were mixed with an equal volume of Laemmli buffer (BioRad), heated to 95°C for 5 min and then chilled on ice. The samples were loaded in NuPage 4–12% 1.5 × 15 well gels (ThermoFisher Scientific) and subjected to electrophoresis using 200 V, 125 mA for 25 min. The gels were stained with CBB on a rocking platform for 30 min and destained using a solution of Milli-Q water, methanol, and acetic acid in a ratio of 50/40/10.
Transmission electron microscopy
Three microlitres of exosome samples (10 ng/µL) were added onto dental wax. Three hundred mesh formvar-carbon coated copper grids were put onto the droplet for 20 min. The grids were transferred onto 2% uranyl acetate and incubated for 1 min. Next, the grids were washed five times with distilled water and blotted dry with filter paper. The grids were then imaged using a transmission electron microscope (H7650, Hitachi, Tokyo, Japan). Each image was taken at 8000 × magnification and 30,000 × magnification.
In vitro studies
Fibroblast cell culture
Normal human dermal fibroblasts (NHDF CC-2509, Lonza, Basel, Switzerland) were maintained and expanded in Dulbecco’s Modified Eagle Medium with 4.5 g/L glucose (Sigma-Aldrich) supplemented with 10% foetal bovine serum (Japan Bioserum, Hiroshima, Japan) and 1% antibiotic (Penicillin-Streptomycin, Wako). The cells were stored in CellBanker 1 (Amsbio, Abingdon, UK).
Fibroblast proliferation assay
NHDFs were seeded at passage 13 to a 96-well plate at a density of 5000 cells/cm2 in serum containing media. The following day, the media was replaced with serum-free media with or without exosomes. Seventy-two hours later, the cell number was determined by cell counting kit (CCK-8, Dojindo, Tokyo, Japan), according to the manufacturer’s instructions.
ELISA, Cytotoxicity assay and qPCR
NHDF passage 15 was seeded at 25.000 cells/cm2 in 24-well plates and left to adhere overnight. The following day the media was changed to serum-free media (900 µL/well) including 2 µg/mL of either cExo or ncExo. Serum-free media only and 10 nM dexamethasone supplement were used as controls. The media was collected after 72 h, frozen in −80°C degree freezer and later analysed using HGF ELISA (ab100534, Abcam), according to the manufacturer’s instruction. The cell number was determined by cell counting kit (CCK-8, Dojindo), according to the manufacturer’s instructions. The cells were then lysed using RLT-buffer (Qiagen) with 1% beta-mercaptoethanol (Wako), and the lysate stored in −80°C. RNA was then isolated using RNEasy kit (Qiagen, Hilden, German) according to the manufacturer’s instructions, quality and quality read on spectrophotometer (NanoDrop ND 2000, ThermoFisher Scientific), and cDNA was synthesized (NP100042, Origene, MD, USA). qPCR was performed using TaqMan Fast Advanced Master mix in StepOnePlus-plates and the following probes; beta actin as housekeeping gene (4326315E-1,112,022, applied biosystem), hgf (hs00300159_m1), vegfa (hs00900055_m1), fgf2 (hs00266645_m1), ctgf (hs01026927_g1), all from ThermoFisher Scientific. Analysis was made using 2–∆∆Ct method.
Bacterial growth assay
Staphylococcus aureus (ATCC25723) was precultured in Mueller-Hinton (MH) broth at 37°C for 18 h under aerobic conditions. The bacterial suspension was diluted to 106 colony-forming units per mL (CFU/mL) with the medium. The portion of the bacterial suspension (105 CFU) was inoculated into 96-well plates containing or without 2 µg/mL of the exosome samples and incubated for 0, 3, 6, 9 and 24 h, respectively. Each appropriate concentration of bacterial suspension from the 96-well plates was seeded to the MH agar. Colony-forming units (CFU) was then counted after incubation at 37°C for 24 h aerobically.
Ex vivo and in vivo assays
Exosome labelling using fluorescent dye
Exosomes were labelled using fluorescent dye (PKH26, Sigma-Aldrich); 20 µg exosomes were diluted in 500 µL Diluent C. Two microlitres of PKH26 were added to another 500 µL Diluent C and the two solutions were rapidly added together and mixed by pipetting for 5 min, after which 2.5 mL PBS was added and then transferred to a 10 kDa filter and centrifuged at 7500 × g, 4°C for 5 min. To wash out unbound dye, the filtrate was discarded and fresh PBS added and centrifuged again. This was done in total three times. As a control, the same procedure was performed but without exosomes. Successful staining was confirmed by visualizing the samples under fluorescent dissection microscope (BX51, Olympus, Tokyo, Japan).
Ex vivo pig oesophageal wound adhesion assay
Oesophageal tissue was excised from a pig sacrificed in an unrelated experiment. Mucosal wounds were made using 5 mm biopsy punches (Kai medical, Tokyo, Japan), to simulate submucosal dissection. Stained exosomes (2 x cExo, 1 x ncExo) or control suspension (as prepared according to previous paragraph) were added to the wound bed and left to adhere for 1, 2 or 5 min. The wound bed was then submerged and washed in PBS to remove unbound exosomes. The tissue was observed under fluorescent dissection microscope (Olympus). All microscopy settings were kept the same throughout the experiment except for an increased exposure time at 1 min, due to weak signal in that group.
Rat full-thickness skin wound model and histopathology
Animal experiments were performed after approval from the Animal Welfare Committee of Tokyo Women’s Medical University School of Medicine. Ten adult Sprague Dawley rats (body weight 248 ± 26 g, Japan SLC Inc. Shizuoka, Japan) were used. The animals were sedated using isoflurane (Pfizer, NY, USA) and anesthetized by a combination of medetomidine (Domitor, Nippon Zenyaku Kogyo, Fukushima, Japan, 0.1875 mg/kg), Midazolam (Sandoz, Japan, 1 mg/kg) and Butorphanol tartrate (Meiji Seika, Japan, 1.25 mg/kg) by subcutaneous injection. Dorsal fur was shaved with electronic trimmer and hair removed by depilatory cream. After cleaning, four full-thickness wounds were made using 5 mm biopsy punches (Kai medical). Exosomes (cExo and ncExo, n = 9–10 wounds), control solution (as described under “Exosome labeling using fluorescent dye”-heading, n = 4 wounds) and PBS (n = 6 wounds) was topically added to the wounds. To adjust for any anatomical differences that could affect the wound healing, the substances were rotated between each animal. After adding the substances, they were left for 30 min to allow adhesion, after which the wounds were covered with TegaDerm (3M) and gauze. We tried two administration protocols: either 7.6 µg exosomes spread over day 0 and day 1, or 12.5 µg exosomes on day 0. The animals were observed daily and sacrificed on day 6 or 17. The wounds were excised using 8 mm biopsy punches (Kai medical), cut in half, frozen in OCT compound (Sakura Finetek Japan, Tokyo, Japan) and 8-µm sections were made on a cryostat (Hyrax C50, MicroEdge). The sections were stained using Hematoxylin-Eosin (H&E) and Picrosirius red. The H&E slides were visualized under light microscopy (Eclipse E800, Nikon, Tokyo, Japan) while the Picrosirius red slides were visualized under a polarized microscope (TE 2000–4 with T-A2, Nikon). Sections were also visualized using a confocal microscopy (Fluoview FV1200, Olympus) to identify signal from labelled exosomes. The wound width was measured using ImageJ (National Institute of Health, MD, USA) in a blinded fashion by two independent researchers who were instructed to measure three lines per image; high, middle and low position of the dermis (see supplemental Figure 1(a)).
Statistical analysis
Graphs are presented as ± standard deviation. Graph Pad Prism version 5 (GraphPad Software, CA, USA) was used for statistical analysis. Student’s t-test or analysis of variance with Tukey’s post-test was used and statistical significance indicated as: *P ≤ 0.05, **P ≤ 0.01 and ***P ≤ 0.001.
Results
Exosome isolation and characterization
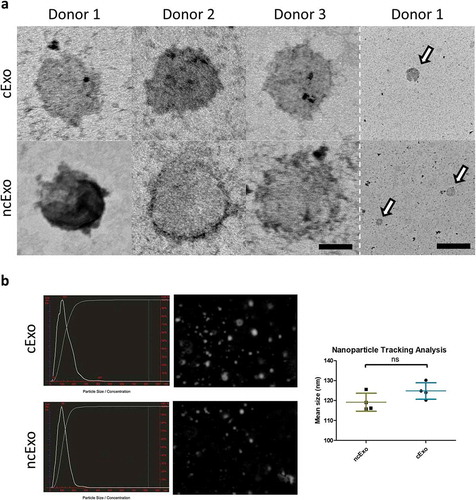
details the eight healthy human donors, cell viability and purity. The exosome protein yield per millilitre media was significantly lower in the conditioned group (cExo) compared to non-conditioned group (ncExo) (). There was no significant difference between number of particles isolated per millilitre media as well as amount of protein per particle between the two groups (). We evaluated the amount of proteins from different fractions of the size-exclusion chromatography and found that the bulk proteins diluted in the “non-exosome”-fractions (fractions >10), as expected for both cExo () and ncExo (supplemental Figure 1(b)). Total protein staining of electrophoresis gels indicated that the exosome-fractions (f7–9) contained minimal amount of small molecules and the majority of its proteins were over 100 kDa (). Western blots showed that both cExo and ncExo were positive for CD9 (). Also Flotillin-1 was positive in both groups (). Proteins commonly found in other exosome preparations (Annexin V, EpCAM and HSP70) were negative in both groups (). GRP94, a protein that suggests cellular contamination of the exosomes, was negative in both groups (). The non-exosomal fraction (f14) was negative for all markers, while the positive control (human oral keratinocyte-cell lysate) was positive for all markers except for EpCam (). Transmission electron microscopy (TEM) revealed spherical morphology and similar sizes of vesicles in both groups (). Nanoparticle tracking analysis showed a mean diameter of 119.2 ± 4.5 nm for ncExo and 124.8 ± 4.1 nm for cExo, with no statistically significant difference between the groups ().
Figure 2. Donor details and exosome isolation. (a) Details of healthy volunteers which have donated blood and oral mucosal tissue, and the outcome of cell isolation. (b) The yield, expressed as nanogram protein per millilitre media, was higher in non-conditioned media than in the conditioned media (n = 7). (c) The number of isolated particles per millilitre media (d) and the amount of protein per particle (e) was not significantly different between the groups (n = 3). (e) Protein quantification of different fractions from the size exclusion chromatography. Fractions 7–9 (“EV-fractions”) had considerably lower protein content, suggesting a successful separation from bulk non-EV-proteins. (f) Coomassie Brilliant Blue-staining of gel electrophoresis suggests that fractions 7–9 are almost completely void of contaminating proteins. The dotted rectangle indicates albumin (66.5 kDa), a common contaminant.
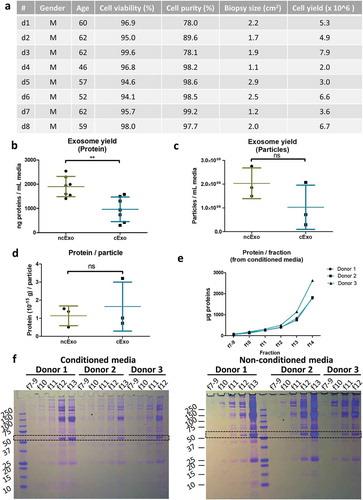
Figure 3. Western blot analysis. Western blot was performed on cExo and ncExo samples, both from exosome-fractions (fractions 7–9) and from a non-exosome-fraction (fraction 14). Normal human keratinocyte lysate was used as control. (a) CD9 was positive in all exosome-samples and in the lysate, but negative in the non-exosome-fraction. (b) Flotillin was positive in all exosome-fractions and lysate, but with varying signal strength, and no signal was detected in the non-exosome-fraction. (c) Annexin V was negative in all fractions, but positive in the lysate. (d) EpCAM was negative in all samples (expected at 40 kDa, dotted box). (e) HSP70, heat shock protein 70, was negative in all samples except for cell lysate control. (f) GRP94, an endoplasmic reticulum protein that commonly contaminates vesicle isolates, was also negative in all samples except for cell lysate control. d3, d4 and d5 refer to donors 3, 4 and 5.
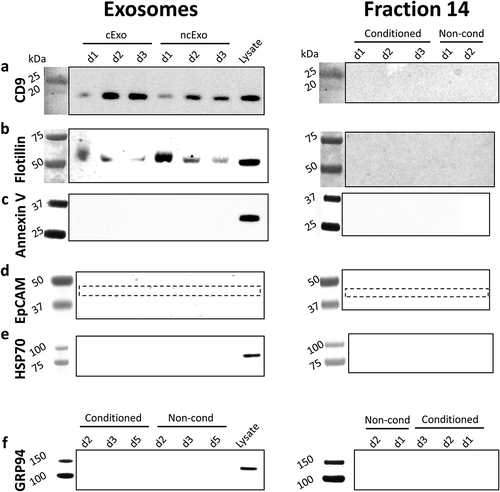
Figure 4. Electron microscopy and nanoparticle tracking analysis. (a) Transmission electron microscopy revealed a rounded appearance from both groups of exosomes. (b) Nanoparticle tracking analysis showed a mean size of around 125 nm with no difference between the two groups (n = 4). Scale bar = 50 nm (left) and 500 nm (right).
In vitro studies using fibroblasts
We decided to use human fibroblasts as our target cells in in vitro assays due to their importance in fibrosis and stricture formation. Since corticosteroids are used clinically to prevent strictures, we used dexamethasone as a control. First, we investigated how the exosomes affected the proliferation of fibroblasts in a 72-h assay. cEXo showed a dose-dependent reduction of fibroblast proliferation, 2 µg/mL was significantly different to 0.2 µg/mL, both to a degree not different from dexamethasone. In contrast, non-exosome-fraction (f12), from the conditioned media did not significantly reduce the proliferation. ncExo also significantly reduced fibroblast proliferation, but to a lesser extent, and only at 2 and 0.7 µg/mL. Similarly, the non-exosome-fraction from this group did not reduce proliferation but instead stimulated proliferation at 0.7 µg/mL ().
Figure 5. In vitro bioactivity. (a) The exosomes were tested in a 72-h proliferation assay. At 0.2 µg/mL, cExo, but not ncExo significantly reduced the proliferation of cells, to the same extent as dexamethasone. At higher concentrations the difference between the groups was less (n = 12 for EV groups, n = 4–8 for control groups, * indicates significance difference to 0 µg/mL, † indicates significant difference to dexamethasone group). (b) Neither of the exosome groups nor dexamethasone showed any significant cytotoxicity (72 h with high density seeding, 2 µg/mL). (c) The gene expression of several wound-healing related genes was up-regulated after exposure to both types of exosomes. In general, cExo induced the expression more strongly than ncExo, but this was only significant for HGF. Dexamethasone suppressed the expression for all factors except for CTGF. (d) Stimulation with both groups of exosomes (2 µg/mL) led to an extensive increase of HGF-secretion from fibroblasts. (e) cExo had a small suppressive effect on S. aureus at the 3-h time point (2 µg/mL).
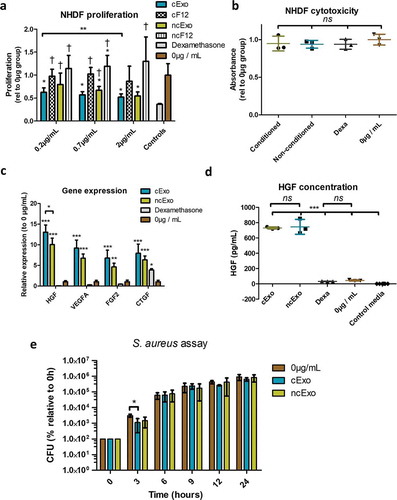
We proceeded with a 72 h cytotoxicity assay, where exosomes were added to non-proliferative (confluent) fibroblasts. Neither cExo, ncExo, nor dexamethasone showed any cytotoxic effects (). The gene expression of these cells was subsequently evaluated using qPCR for genes important for wound healing and tissue regeneration: hepatocyte growth factor (HGF), vascular endothelial growth factor A (VEGFA), fibroblast growth factor2 (FGF2) and connective tissue growth factor (CTGF). Both cExo and ncExo significantly upregulated these genes as compared to 0 µg control and dexamethasone group. For HGF, cExo showed a significantly higher induction than ncExo; in the other factors, there was a non-significant trend towards higher induction in cExo. For dexamethasone, there was a trend towards decreased expression for all factors except for CTGF where there was a significant increase (). We confirmed the increase of HGF on protein level by examining the conditioned media with ELISA, revealing an approximate 16-fold increase in released HGF ().
Bacteriological studies
Given the known antibacterial effects of peptides released from the oral mucosa, we hypothesized that exosomes derived from oral keratinocytes exhibit similar properties. We performed a colony-forming assay using S. aureus and exosomes at a concentration of 2 µg/mL. The exosomes showed a weak, but significant, reduction of growth at the 3-h time point by cExo, but not by ncExo ().
Ex vivo pig oesophageal wound adhesion assay
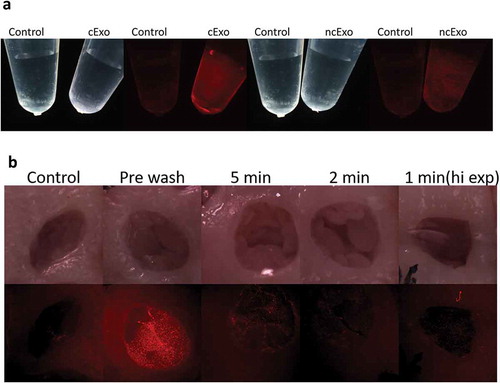
The exosomes could successfully be labelled with membrane-binding fluorescent dye with minimal signal from the control (). To evaluate the adhesion of exosomes to oesophageal wounds, we used an ex vivo model, where mucosal wounds were made on freshly dissected oesophageal tissue. The tissue was imaged using a dissection microscope with fluorescent light capability. Control tissue (without exosomes) showed a weak auto-fluorescence from the mucosa. A strong signal was detected after topical application of the exosomes (“pre wash”, ). We first tried a 5-min adhesion time: the exosomes were added and after 5 min the tissue was submerged and washed in PBS to eliminate non-bound exosomes. The signal was clearly detectable and we proceeded with a 2-min adhesion time after which signal could still be detected, but evidently weaker. Even after 1-min adhesion time, a signal could be detected but only at an increased exposure time. Taken together, the exosomes appear to adhere rapidly and strongly to the oesophageal wound bed.
Figure 6. Fluorescence labelling and adhesion assay. (a) Exosomes from both groups could successfully be labelled with membrane-binding fluorescence dye. (b) Adhesion was tested in an ex vivo model using porcine oesophagus. The control tissue showed a weak auto fluorescence. Labelled exosomes were topically added and the tissue visualized under dissection microscope (“pre-wash”). The exosomes were allowed to adhere for 5, 2 or 1 min, after which the tissue was washed to remove unbound exosomes. The signal decreased with shorter adhesion time. After 1-min adhesion time, the signal could still be detected, but only with increased exposure time.
In vivo full-thickness wound model in rat
We used 5-mm biopsy punches to create uniform, full-thickness wounds on the dorsal site of the rat, resulting in wounds with diameters of 0.19 ± 0.03 cm2 (n = 12, SD). All animals tolerated the model well, with a maximum weight loss of 4% and full recovery of weight in all animals by the sixth day (supplemental Figure 1(c)).
Exosome tracking in vivo
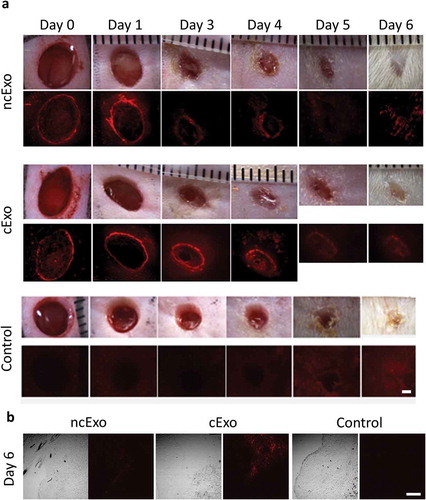
We topically applied labelled exosomes to the wound bed immediately after creating the wound and on the subsequent day. Each wound was observed under a fluorescent microscope to track the exosomes. However, starting from d4-d5 overlying fibrin scab and hair re-growth hampered both accurate measurements of wound size (due to difficulty identifying the wound margin) and fluorescent signal (due to auto florescence of the fibrin scab) (). Positive signal could be confirmed under dissection microscope until day 4. After histological sectioning of frozen tissue samples, positive signal could be found in 3/3 cExo and 2/3 ncExo using confocal microscopy ().
Figure 7. Exosome tracking in vivo. (a) Labelled exosomes were topically applied to full-thickness wounds on rat dorsal sites. The exosomes were added just after the wound was made and on the following day. The signal could be detected up to day 4. On days 5 and 6 it was difficult to distinguish the signal due to auto fluorescence from re-grown hair and fibrin scab. (b) Using confocal microscopy on sections from the wounds at day 6, signal could be detected in 2/3 ncExo and 3/3 cExo samples. Scale bar = 100 µm.
Histopathological analysis of wounds
We evaluated two different topical application protocols: (1) 7.6 µg spread over day 0 and day 1, and (2) 12.5 µg on day 0 only. There was no considerable difference between the macroscopic wound size areas as measured from digital images. H&E staining revealed that the granulation tissue was decreased in cExo group using protocol 1 (). Picrosirius staining visualized under polarized light revealed that a larger part of the cExo wounds had regained a more normal collagen distribution (). Measurements of wound size based on Picrosirius red staining were performed by two blinded researchers. cExo using protocol 1 showed the highest wound healing capacity, significantly lower than all other treatment groups ().
Figure 8. cExo stimulate wound healing. Two protocols were tried. In protocol 1, 7.6 µg exosomes were applied over the first 2 days. Protocol 2 involved applying 12.5 µg exosomes on day 0 only. (a) For protocol 1, H&E staining revealed a reduced wound size (brackets) and a largely restored histological architecture surrounding the wound area in the cExo group. In contrast, ncExo appears similar to the PBS group. Protocol 2 was considerably less effective with similar histopathology between the groups. (b) Polarized visualization of Picrosirius staining clearly shows the wound area (brackets) with less signal due to minimal collagen content. (c) Quantification of the wound sizes revealed a considerable decrease of wound size using cExo and protocol 1, significant from all other groups. (d) Protocol 1 on a 17-day time point revealed similar results, with a reduced wound size by cExo only, confirmed by blinded quantification (e). Scale bars = 200 µm.
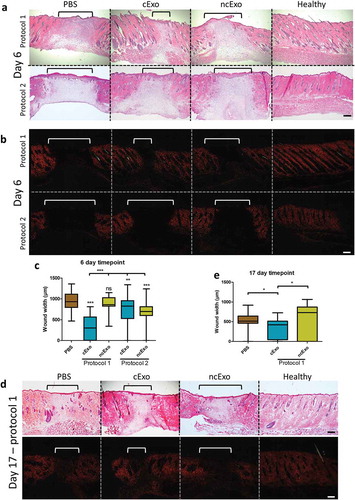
After the promising result from the 6 day-time point using protocol 1, we proceeded with a 17-day time point. The results were similar, with a significant reduction of wound width in the cExo-, but not in the ncExo group (). The hypertrophic epithelium observed in all groups at 6 days () was reduced to a near-normal sized epithelium at the 17-day time point ().
Discussion
In the present study, we have investigated the regenerative potential of exosomes derived from clinical-grade oral mucosa epithelial cell sheets production. To our knowledge, this is the first time that exosomes from this cell source have been studied from a regenerative medicine perspective. We also showed that a waste product (used media) could be transformed into a therapeutic agent.
Cell therapy has gained increasing interest over the last few decades. Although the mode-of-action remains poorly understood, many reports suggest that the effect is attributed to released factors rather than a long-term engraftment of cells. This seems to be especially true for one of the most common cell types for cell therapy, mesenchymal stromal cells (MSCs). For example, MSCs have been used for skin wound healing [Citation18], graft-versus-host disease [Citation19] and bronchopulmonary dysplasia [Citation20]. Interestingly, all three of these indications have been successfully treated with MSC-derived exosomes [Citation21–Citation23]. From a therapeutic point of view, exosomes have several advantages over cells. Their small size enables sterile-filtration and could theoretically reduce the risk of embolism after intravenous injections. Since they are metabolically inactive, there is also no risk of teratoma formation, one of the major concerns with embryonic- or induced pluripotent stem cell therapy.
Cell sheet technology was developed by Okano et al [Citation24]. Using culture dishes or inserts coated with a thermo-responsive polymer, cells can be detached from the underlying material without the need for enzymatic digestion, resulting in contiguous sheets of cells which retain their cell-to-cell contact and deposited extracellular matrix. Cell sheets based on this technology have been used in clinical scenarios for regenerative medicine of the cornea [Citation25], oesophagus [Citation7], periodontal tissue [Citation26] and articular cartilage [Citation27]. Like other cell and tissue engineering therapies, the underlying mode-of-action is not fully understood. Here, we have investigated exosomes derived from clinical-grade cell sheet production for oesophageal regeneration. The primary question was whether the waste products (used or leftover culture media) could be converted to a therapeutic agent to further improve the regeneration of the oesophageal mucosa. Additionally, we hypothesized that this research could shed light on the mode-of-action of cell sheets and on the unique wound-healing capacity of the oral mucosa.
As with many emerging scientific fields, extracellular vesicle (EV) research initially suffered from a lack of standardization regarding characterization and isolation. To the comparability of our results with other researchers, we adhered to the International Society for Extracellular Vesicles minimal requirements for defining EVs [Citation28]. Given our vesicle size of less than 150 nm diameter, we sub-defined them as exosomes according to Tkach et al [Citation29]. To improve transparency and reproducibility, we have also used the relatively new service EV-TRACK [Citation30] to make details on our isolation and characterization studies publicly available (tracking code: YJ7428AP).
We acquired conditioned and non-conditioned media from cell sheet production from healthy donor oral mucosal cells. We received on average ~70 mL of conditioned media and ~35 mL of non-conditioned media. This represents around 20% of all conditioned media produced for one typical oesophageal cell sheet patient (~320 mL, based on 10 sheets for one patient). Around 1 µg of cExos could be isolated from 1 mL of conditioned media, meaning that ~320 µg autologous cExo potentially could be produced for one patient. Our finding that the exosome yield (based on amount of protein) from conditioned media was lower than from non-conditioned media ()) is somewhat counterintuitive. The particle count showed a similar trend ()). We expected the yield to increase as the keratinocyte released exosomes into the media. This might be explained by internalization or degradation of the serum exosomes during the cell sheet culture.
We used in vitro assays using fibroblasts to learn more on the bioactivity of the two groups of exosomes compared to the corticosteroid dexamethasone as a control. In general, cExo and ncExo showed similar characteristics. Both groups reduced the proliferation of fibroblasts similarly to dexamethasone. cExo appeared to be more potent: as a lower concentration (0.2 µg/mL), cExo could still exert the same effect as dexamethasone. The “non-exosome” fractions did not significantly decrease the proliferation from either of the groups, which is in line with other studies which showed that exosomes, but not other fractions of the secretome, could reduce myocardial injury [Citation10]. Another approach to preventing oesophageal strictures is by mitomycin C, which also inhibits fibroblast proliferation [Citation31]. Thus, we believe that our in vitro findings showing the suppression of fibroblast growth are promising for the final clinical application. Moreover, we found that both cExo and ncExo (but cExo to a larger extent) stimulated the release of several growth factors important for tissue regeneration. We found a small inhibitory effect of cExo on S. aureus growth. This is probably of small importance for our potential clinical application but could be worth studying for other applications.
Although the optimal way to deliver exosomes to an oesophageal wound is not determined, adhesion to the wound bed is likely key to a future successful clinical outcome. In an ex vivo model using pig oesophagus we could detect signal from fluorescently labelled exosomes after as little as 1 min of adhesion time.
For an in vivo model, we decided to use full-thickness skin wounds in rats. Oesophageal wounds would have better reflected the clinical scenario, but considering the limited amount of exosomes we could receive, it was not feasible for this study and would also have caused more suffering to more animals. For this reason we decided to use a skin model where we could more easily create uniform wounds by biopsy punch, and have controls and samples in the same animal, thus reducing inter-animal variability. Interestingly, our results indicate that repeated administration is more important than dose, since 12.5 µg on day 0 was less effective than 7.6 µg administered over the first 2 days. We found a stimulation of wound healing on both 6-day and 17-day time point, even in this xenogeneic setting. The effect is likely to be even stronger in an allogeneic or autologous setting. Similarly to our findings, other researchers have recently shown that EVs isolated from saliva can promote haemostasis, the first phase of wound healing [Citation32].
This study has some evitable and inevitable weaknesses. First, while ncExo consists of exosomes derived from human serum, cExo consists of a mixture of OMEC-derived- and serum-derived exosomes. Generally, it is common to use exosome-free culture conditions (for example, serum-free media) when producing exosomes. However, the main purpose of this study was to evaluate if waste from clinical-grade cell sheet production could be turned into a therapeutic agent aimed for autologous use – meaning that we had to adhere to the established production protocols. Second, we used a fluorescent membrane-binding dye to track exosomes ex vivo and in vivo. Understandably, positive signal is not a definite proof of maintained exosomes as the fluorescence might come from released dye from degraded vesicles. Lastly, these experiments were performed over approximately 1.5 years and the time in −80°C freezer and the number of freeze/thaw cycles for each sample was not controlled. We did not detect any difference in our assays depending on the storage time or freeze/thaw cycles, but this needs to be more carefully studied. Lastly, the donors were all adult males, aged 46–62 years old. It would have been interesting to evaluate if there are any differences related to donor gender and age, but such comparison was not performed during this study.
In conclusion, we provide a proof-of-concept that waste from clinical-grade cell sheet production can successfully be transformed into a therapeutic agent that stimulates wound healing. To our knowledge, this is a unique concept which can be expanded to other applications. This is also likely the first time that exosomes are isolated from OMECs in order to study their pro-regenerative potential.
Geolocation information
This work was performed in Tokyo and Iwate, Japan and in Stockholm, Sweden.
Supplemental Material
Download TIFF Image (1.9 MB)Acknowledgments
This work was supported by The Swedish Society of Medicine, Erik och Edith Fernströms stiftelse för medicinsk forskning, Misao-Yanagihara-Grant for regenerative medicine research and JSPS KAKENHI Grant Number JP18H02985. We thank Dr Takahashi and Mr Okada at The Cancer Institute, Japanese Foundation for Cancer Research for help with Nano Tracking Analysis. We also thank Mr Kinji Ishida and Tomohito Hanasaka at Technical Support Center for Life Science Research (LSR) of Iwate Medical University, Iwate, Japan for the help with TEM observations. Finally, we are grateful to CellSeed Inc for providing the clinical-grade media.
Disclosure statement
Tokyo Women’s Medical University has a pending patent regarding the use of oral mucosa-derived exosomes with SS, NK and T Iwata as inventors
Supplementary material
Supplemental data for this article can be accessed here
Additional information
Funding
References
- Mak K, Manji A, Gallant-Behm C, et al. Scarless healing of oral mucosa is characterized by faster resolution of inflammation and control of myofibroblast action compared to skin wounds in the red Duroc pig model. J Dermatol Sci. 2009;56:168–16.
- Board-Davies E, Moses R, Sloan A, et al. Oral mucosal lamina propria-progenitor cells exert antibacterial properties via the secretion of osteoprotegerin and haptoglobin. Stem Cells Transl Med. 2015;4:1283–1293.
- Khurshid Z, Naseem M, Sheikh Z, et al. Oral antimicrobial peptides: types and role in the oral cavity. Saudi Pharm J. 2016;24:515–524.
- Leavitt T, Hu MS, Marshall CD, et al. Scarless wound healing: finding the right cells and signals. Cell Tissue Res. 2016;365:483–493.
- Viklund P, Lindblad M, Lu M, et al. Risk factors for complications after esophageal cancer resection: a prospective population-based study in Sweden. Ann Surg. 2006;243:204–211.
- Ohki T, Yamato M, Murakami D, et al. Treatment of oesophageal ulcerations using endoscopic transplantation of tissue-engineered autologous oral mucosal epithelial cell sheets in a canine model. Gut. 2006;55:1704–1710.
- Ohki T, Yamato M, Ota M, et al. Prevention of esophageal stricture after endoscopic submucosal dissection using tissue-engineered cell sheets. Gastroenterology. 2012;143:582–588.e2.
- Yamaguchi N, Isomoto H, Kobayashi S, et al. Oral epithelial cell sheets engraftment for esophageal strictures after endoscopic submucosal dissection of squamous cell carcinoma and airplane transportation. Sci Rep. 2017;7:1–12.
- Jonas E, Sjöqvist S, Elbe P, et al. Transplantation of tissue-engineered cell sheets for stricture prevention after endoscopic submucosal dissection of the oesophagus. United Eur Gastroenterol J. 2016;4:741–753.
- Lai RC, Arslan F, Lee MM, et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010;4:214–222.
- Tan JL, Lau SN, Leaw B, et al. Amnion epithelial cell-derived exosomes restrict lung injury and enhance endogenous lung repair. Stem Cells Transl Med. 2018;7:180–196.
- Tsujii Y, Hayashi Y, Kawai N, et al. Risk of perforation in balloon dilation associated with steroid injection for preventing esophageal stricture after endoscopic submucosal dissection. Endosc Int Open. 2017;5:E573–E579.
- Larjava, H,Wiebe C, Gallant-Behm C, et al. Exploring scarless healing of oral soft tissues. J Can Dent Assoc (Tor). 2011;77:1–5.
- Dereli M, Krazinski BE, Ayvaz S, et al. A novel approach for preventing esophageal stricture formation: olmesartan prevented apoptosis. Folia Histochem Cytobiol. 2014;52:29–35.
- Kerwin LY, El Tal AK, Stiff MA, et al. Scar prevention and remodeling: A review of the medical, surgical, topical and light treatment approaches. Int J Dermatol. 2014;53:922–936.
- Chhabra S, Chhabra N, Kaur A, et al. Wound healing concepts in clinical practice of OMFS. J Maxillofac Oral Surg. 2016;16:403–423.
- Hans M, Madaan Hans V. Epithelial antimicrobial Peptides: guardian of the oral cavity. Int J Pept. 2014;2014:1–13.
- Isakson M, de Blacam C, Whelan D, et al. Mesenchymal stem cells and cutaneous wound healing: current evidence and future potential. Stem Cells Int. 2015;2015:831095.
- Ringdén O, Uzunel M, Rasmusson I, et al. Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplantation. 2006;81:1390–1397.
- Augustine S, Avey MT, Harrison B, et al. Mesenchymal stromal cell therapy in bronchopulmonary dysplasia: systematic review and meta-analysis of preclinical studies. Stem Cells Transl Med. 2017;6:2079–2093.
- Wang L, Hu L, Zhou X, et al. Exosomes secreted by human adipose mesenchymal stem cells promote scarless cutaneous repair by regulating extracellular matrix remodelling. Sci Rep. 2017;7:1–12.
- Kordelas L, Rebmann V, Ludwig AK, et al. MSC-derived exosomes: a novel tool to treat therapy-refractory graft-versus-host disease. Leukemia. 2014;28:970–973.
- Willis GR, Fernandez-Gonzalez A, Anastas J, et al. Mesenchymal stromal cell exosomes ameliorate experimental bronchopulmonary dysplasia and restore lung function through macrophage immunomodulation. Am J Respir Crit Care Med. 2018;197:104–116.
- Yamato M, Okano T. Cell sheet engineering. Mater Today. 2004;7:42–47.
- Nishida K, Yamato M, Hayashida Y, et al. Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. N Engl J Med. 2004;351:1187–1196.
- Iwata T, Yamato M, Washio K, et al. Periodontal regeneration with autologous periodontal ligament-derived cell sheets – a safety and efficacy study in ten patients. Regener Ther. 2018;9:38–44.
- Sato M, Yamato M, Hamahashi K, et al. Articular cartilage regeneration using cell sheet technology. Anat Rec. 2014;297:36–43.
- Lötvall J Hill AF, Hochberh F, et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the international society for extracellular vesicles. J Extracell Vesicles. 2014;3:26913.
- Tkach M, Théry C. Communication by extracellular vesicles: where we are and where we need to go. Cell. 2016;164:1226–1232.
- Van Deun J, Mestdagh P, Agostinis P, et al. EV-TRACK: transparent reporting and centralizing knowledge in extracellular vesicle research. Nat Methods. 2017;14:228–232.
- Bhatnagar V, Kumar A. Topical application of mitomycin-C in corrosive esophageal strictures. J Indian Assoc Pediatr Surg. 2005;10:25.
- Yu Y, Gool E, Berckmans RJ, et al. Extracellular vesicles from human saliva promote hemostasis by delivering coagulant tissue factor to activated platelets. J Thromb Haemost. 2018;16:1153–1163.
