ABSTRACT
The prevalent porcine helminth, Ascaris suum, compromises pig health and reduces farm productivity worldwide. The closely related human parasite, A. lumbricoides, infects more than 800 million people representing a disease burden of 1.31 million disability-adjusted life years. The infections are often chronic in nature, and the parasites have a profound ability to modulate their hosts’ immune responses. This study provides the first in-depth characterisation of extracellular vesicles (EVs) from different developmental stages and body parts of A. suum and proposes the role of these vesicles in the host–parasite interplay. The release of EVs from the third- (L3) and fourth-stage (L4) larvae and adults was demonstrated by transmission electron microscopy (TEM), and sequencing of EV-derived RNA identified a number of microRNAs (miRNAs) and transcripts of potential host immune targets, such as IL-13, IL-25 and IL-33, were identified. Furthermore, proteomics of EVs identified several proteins with immunomodulatory properties and other proteins previously shown to be associated with parasite EVs. Taken together, these results suggest that A. suum EVs and their cargo may play a role in host–parasite interactions. This knowledge may pave the way to novel strategies for helminth infection control and knowledge of their immune modulatory potential.
Introduction
The large roundworm, Ascaris suum, is one of the most important and prevalent parasitic nematodes of pigs. Although mostly subclinical, infections are associated with production losses due to impaired nutrient utilisation and reduced weight gain, but pigs may also be affected by a high worm burden [Citation1]. In addition, A. suum infection may increase the pathogenicity of bacterial co-infections and compromise vaccine efficacy [Citation2]. A. lumbricoides is highly prevalent in humans and 819 million people are estimated to be infected [Citation3]. Due to the high genetic relatedness between A. suum and A. lumbricoides and the pronounced similarities in host physiology, A. suum infections in pigs can be used as model system for studying A. lumbricoides infections in humans [Citation4].
Helminths, including Ascaris spp., induce a Th2-type immune response in their host, characterized by an increased production of eosinophils as well as an elevated secretion of IL-4 and IL-13 by T helper cells [Citation5], and IL-25 and IL-33 by intestinal epithelial cells [Citation6]. An increased level of IL-10 has also been observed in the intestine, where it acts as regulator of inflammation [Citation7]. In addition, a strong suppression of both Th1 and Th17 immune function is a typical outcome of helminth infections [Citation8] and T cell function is impaired in mice by A. suum products [Citation9]. Dendritic cells (DCs) play an important role in the initiation of immune responses, as they induce polarization of naïve T-cells to Th cell populations, and modulation of DCs by parasite-derived products has been suggested to, at least partly, be responsible for the suppression of Th1/Th17 responses. For example, A. suum inhibit DC activation [Citation10] and direct contact between DCs and specific helminth products, such as schistosome soluble egg antigens [Citation11] results in a reduced production of pro-inflammatory cytokines [Citation12]. In addition, we have recently shown that A. suum body fluid (ABF) has a strong overall immunosuppressive effect on DCs including inhibition of pro-inflammatory lipopolysaccharide (LPS)-induced TNF-α and IL-6 secretion [Citation13]. Similarly, ABF has a pronounced suppressive effect on the secretion of TNF-α, IL-6 and IL-10 from LPS-stimulated classically activated human macrophages [Citation14]. The active constituents of the ABF responsible for the suppressive effect on DCs and macrophages have not yet been identified.
Extracellular vesicles (EVs) are membrane-enclosed nanoparticles that can be released from all kinds of organisms, ranging from single-celled protozoans to multicellular mammals, and from various types of cells [Citation15]. The best described EVs are exosomes (30–100 nm) and microvesicles (100–1000 nm) which play an important role in cell-cell communication as vehicles for molecules and biological signals to be shuttled between cells [Citation16]. Several parasite species have been shown to release EVs that can fuse with host cells and this has been suggested to be a strategy to deliver molecules that can modulate the host immune response or transfer of virulence factors [Citation17,Citation18] For example, suppression of pro-inflammatory TNF-α and IL-6 following the uptake of EVs secreted by the parasitic nematode Heligmosomoides polygyrus in macrophages has been demonstrated recently by Coakley et al. [Citation19] and exosomes co-inoculated with the protozoan parasite, Leishmania into the mammalian host by sand flies during feeding enhance virulence by increasing the production of inflammatory cytokines [Citation20].
One important constituent of EVs are miRNAs, a class of non-coding RNAs of about 22 nucleotides in length [Citation21]. miRNAs are post-transcriptional gene-regulators that target complementary mRNA molecules by inhibiting (or occasionally up-regulating) their translation into protein [Citation22,Citation23]. In this way, miRNAs are involved in a wide range of biological processes including functional immune responses [Citation24]. Recent studies have suggested that EV-associated miRNAs are instrumental for host–parasite interactions in helminths [Citation21,Citation25], and in the seminal study by Buck et al., also their impact on the murine immune response was demonstrated [Citation26]. Besides miRNAs, EVs contain a variety of proteins, including the surrounding membrane, which also have been suggested to play a role in numerous aspects of host–parasite communication including pathogenesis and immune evasion [Citation27,Citation28].
Improved fundamental knowledge about the mechanisms underlying the ability of parasites to establish and survive in the hostile environment of their hosts will potentially also provide clues on how to advance diagnostic methods, vaccines and drugs, which is essential for improved control. Therefore, the study aimed to examine, in detail, EV secretion of A. suum in different life stages and body parts, characterize their protein- and miRNA composition and evaluate the potential role of parasite EVs in host immunomodulation.
Materials and methods
For a full and detailed description see supplemental materials and methods.
In brief, to obtain A. suum L3, unembryonated eggs were isolated from the uterus of female worms and embryonated at 20°C for 2 months. Eggs were mechanically stimulated to hatch according to in-house procedures and incubated for one day at 37°C (5% CO2) in RPMI-1640 (Gibco®) containing Antibiotics-Antimycotics (RPMI+AA) (Gibco®). A. suum L4 were obtained from the small intestine of pigs, necropsied 14 days after inoculation with a single dose of 5,000 infective eggs by the agar migration method [Citation29]. After collection, the larvae were washed four times in HBSS followed by four times in RPMI+AA and Gentamicin (Genta) (Gibco®). The larvae were then incubated for 3 days at 37°C (5% CO2) and the RPMI-AA-Genta was exchanged and collected approximately every 24 h. The Danish Animal Experiments Inspectorate (Permission 2015-15-0201-00760) approved the experiment and procedures. Live adult A. suum were collected from pigs at the Danish Crown slaughterhouses in Ringsted and Herning, Denmark. The worms were washed minimum 6 times (37°C) and incubated at 37°C (5% CO2) in RPMI-AA-Genta, which was exchanged and collected approximately every 24 h. The collected culture media were kept at –80°C until further processing. After 1 and 3 days of incubation a fraction of the worms were dissected and body fluid as well as intestinal tissue was collected.
Extracellular vesicles for RNA sequencing were purified by differential centrifugation including two final ultracentrifugation (UC) steps at 100,000 g according to [Citation30], while EVs for the Nanoparticle Tracking Analysis (NTA) also were obtained using size exclusion chromatography (SEC) by IZON qEV columns (iZON Science Ltd) in accordance to the manufacturer’s protocol. A CM 100 BioTWIN transmission electron microscope was used for visualization of EVs.
NTA was carried out using a Nanosight LM10 (Nanosight, Wiltshire, UK) to measure the concentration and size distribution of particles in the EV samples. In brief, PBS-diluted samples were loaded into the sample chamber using a syringe and the microscope objective was adjusted in order to obtain a clear picture of particles within the beam. For each sample, three measurements were performed with 40s duration of each measurement and the data was analysed using NTA software version 3.1.
Total RNA was isolated from EVs using the miRCURY™ RNA Isolation Kits – Cell & Plant (Exiqon A/S) according to the manufacturer’s protocol. The remaining procedures were carried out at Exiqon A/S, Vedbaek, Denmark, and involved total RNA QC, small RNA library preparation and size selection (18–70 nt). The samples were sequenced using an Illumina HiSeq 2500 run (1 × 50 bp single-end read) (Illumina Inc.,) providing an average of 47 M raw reads per sample. Raw reads were adapter trimmed, Phred quality filtered (Q = 30), length filtered (18–27nt) and mapped to miRBase entries for A. suum using Bowtie 1.2. The reads were then normal transformed across samples using the “normTransform()” function, log2(x + 1), of the DESeq2 package. Differential expression analysis was carried out using DESeq2 and Venn diagrams were prepared with Venny 2.1 for comparison of miRNA compositions. Interactions between A. suum adult ES EV miRNAs and porcine genes were predicted using TargetScan as well as the PITA algorithm [Citation31]. For target-prediction in the pig, all available gene models were downloaded from Refseq (release 84) and corresponding 3ʹ-UTR sequences extracted. For TargetScan only 8mer or 7mer seed type matches and for PITA correspondingly only 8:0:0 or 7:0:0 were considered.
Proteomic analysis of EVs isolated by ultracentrifugation was initiated by a modified filter-aided sample preparation (FASP) for tryptic digestion of proteins (ref 10.1021/acs.jproteome.6b00598). The samples were normalized for identification or label-free quantification and analysed using a UPLC-nanoESI MS/MS setup and the subsequent protein identification and quantitative analysis was performed in MaxQuant 1.5.2.8 by searching the data files against the A. suum reference proteome (Uniprot UP000017900; 9213 protein groups). The results from MaxQuant were statistically analysed in Perseus v1.6.1.1. For quantitative comparison of proteomes, samples were analysed using several independent t-tests with a permutation based false-discovery rate at 5%. Further statistical analysis of the samples included Hierarchical clustering and heatmaps. For visualisation of the uniqueness and differences between sample proteomes, Venn diagrams were created using Venny v2.1. The raw and processed data including statistical information have been made publically available through the ProteomXchange consortia by the reference number PXD009377.
Results
Ascaris suum produces and releases extracellular vesicles in vitro
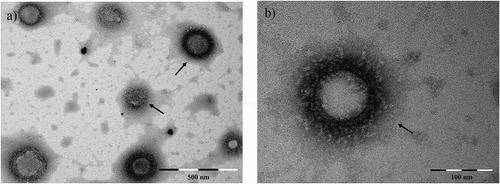
When examining the pellet obtained by UC or SEC by TEM, membrane-enclosed entities with size and features of EVs could be observed in all examined EV samples deriving from A. suum adult worms (), L3, L4, body fluid and intestinal tissue (Supplementary Figure 1), respectively. The structures ranged 80–200 nm in diameter.
Characterization EVs released from Ascaris suum by NTA
In order to examine the EV size distribution and quantify the number of particles, an NTA was carried out using the Nanosight technology. The analysis was conducted for EVs released from A. suum adult worms (Excretory/Secretory (ES) EVs) and EVs obtained from A. suum body fluid (ABF EVs). Both EVs purified by differential centrifugation, i.e. ultracentrifugation (UC), as well as SEC were examined in order to compare the two methods.
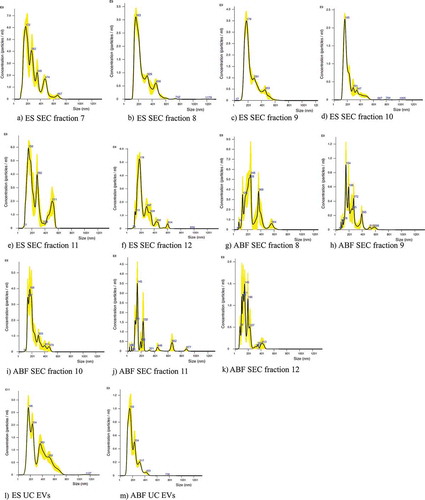
The size of particles in A. suum ES EV samples obtained in SEC fractions 7, 8 and 9 and by UC was in the range of ~210–260 nm (, ). For ABF EV samples, no particles were detected in fraction 7, but in fractions 8 and 9, particles of ~190–220 nm were identified. For both sample types, particles of similar size were also detected in SEC fractions 10, 11 and 12, but in a concentration about 10-fold lower. The number of peaks detected for particles in ES EV and ABF EV samples obtained by SEC were 3–5 and 5–9, respectively, while ES EV and ABF EV samples obtained by UC both had four peaks.
Table 1. The size distribution of particles in extracellular vesicles (EVs) samples based on three readings of each. ES EVs: Excretory/Secretory extracellular vesicles; ABF EVs: Ascaris suum body fluid extracellular vesicles; SEC: Size Exclusion Chromatogaphy; UC: ultracentrifugation. aSee .
Figure 2. Finite track length adjustment (FTLA) Concentration/Size graphs for NTA analysis of particles in A-F) Size Exclusion Chromatography (SEC) fractions 7–12 purified from Ascaris suum Excretory/Secretory (ES) products, G-K) SEC fractions 8–12 purified from A. suum adult body fluid (ABF), L) ultracentrifugation pellet of A. suum ES and M) A. suum ABF.
EVs released throughout parasite development contain miRNA
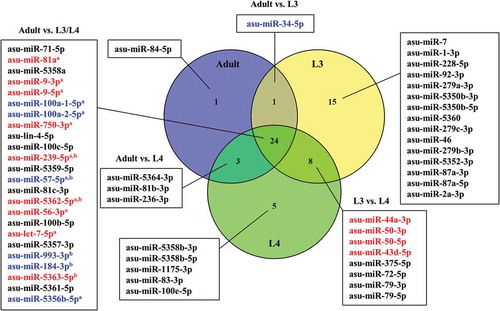
All sequencing datasets contained abundant number of miRNA-reads (2 million reads on average/sample) and following mapping of the processed smallRNAseq data to reference miRNAs available in miRBase we identified 51, 40 and 29 ASU-miRNAs derived from EVs released by A. suum L3, L4 and adult worms, respectively (, Supplementary Table 1). Furthermore, in the supernatant obtained during EV purification of A. suum adult ES-products by UC, 36 miRNAs were identified () and in EVs obtained from ABF and A. suum intestinal tissue, 39 and 42 miRNAs were found, respectively (). The cut-off was set to >100 reads per million.
Figure 4. Venn diagram of miRNAs from Ascaris suum adult Excretory/Secretory (E/S) products associated with extracellular vesicles (EVs) and the EV-depleted supernatant ranked according to average abundance (reads per million). miRNAs shown in blue were down-regulated in A. suum adult ES EVs. None of the common miRNAs were up-regulated.
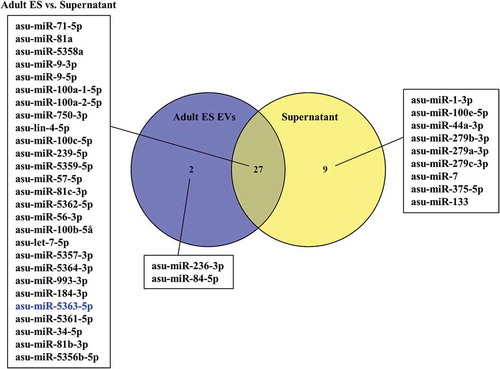
Figure 5. Venn diagram of miRNAs associated with extracellular vesicles obtained from Ascaris suum adult Excretory/Secretory (ES) products, A. suum adult body fluid (ABF) and A. suum intestine ranked according to average abundance (reads per million). miRNAs shown in red were up-regulated and miRNAs shown in blue were down-regulated. aAdult vs. ABF, bAdult vs. Intestine.
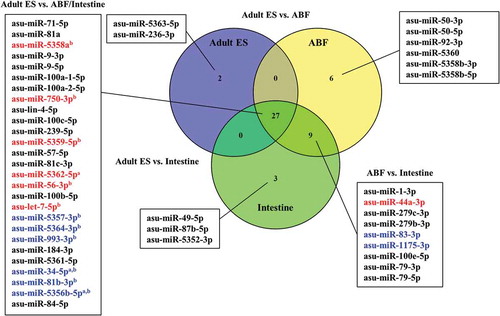
By comparing the composition of EVs from A. suum adults, L3 and L4, the three groups were shown to share 24 miRNAs. Differential expression analysis revealed that out of these common miRNAs, nine were up-regulated in adult EVs compared to EVs from L3 and/or L4, while six were down-regulated (p-value < 0.05, fold change > 2). L3 EVs contained 15 unique miRNAs, whereas L4 contained 5 and in adult EVs only 1 miRNA was unique (). In addition to this, the miRNA composition of A. suum adult ES EVs and adult ES supernatant were compared (). It was found that 27 miRNAs were shared between the 2 groups out of which only 1 miRNA, asu-miR-5363, was significantly down-regulated in the ES EVs compared to the supernatant. The supernatant had nine unique miRNAs, while the ES EVs had two.
ES products, and therefore the EVs in this fraction, may be released from the worms through the anus (intestine) and/or the excretory pore, which is closely linked to the body fluid. Therefore, in order examine which of these is the main site of EV release, the miRNA composition of EVs purified from intestinal tissue and ABF was compared to the miRNA composition of adult ES EVs (). The vast majority (27) of identified miRNAs were shared among all three examined groups. Of these, six miRNAs were significantly up-regulated in adult ES EVs compared to EVs from ABF and/or intestine, while six were down-regulated. Eighteen miRNAs were present exclusively in EVs of ABF and/or intestine while only two were unique for the adult ES EVs.
miRNAs in EVs are predicted to target processes of immunological importance
In order to identify potential target genes of the A. suum miRNAs identified within the present study, a target prediction analysis was carried out. Prior to the analysis, a refined genes-of-interest list was prepared based on a microarray analysis carried out to compare intestinal tissue from pigs infected with A. suum adult worms and naïve, uninfected pigs. Genes were selected if a significant up- or down-regulation had been identified in infected pigs (as detailed in [Citation13]). By PITA and TargetScan analysis, 45/108 genes-of-interest were identified as potential targets of one or more A. suum miRNAs (Supplementary Table 2). Of these, 2 genes had previously been shown to be up-regulated while the remaining 43 genes were down-regulated in infected pigs. Our primary focus was on genes involved in immunity and inflammation, and we identified seven genes of immunological relevance functioning in processes such as T-cell proliferation and activation as well as production of cytokines and chemokines () (the remaining 38 genes are presented in Supplementary Table 2). These genes were all significantly down-regulated in infected pigs. In addition to this, we specifically scanned for targets among genes within the porcine genome encoding hallmark cytokines of helminth infections, i.e. IL-4, IL-10, IL-13, IL-25 and IL-33. From this analysis, IL-13 was identified as a potential target for asu-lin-4-5p and asu-let-7-5p, while asu-miR-5350d-5p may target IL-25 and asu-miR-87a-3p and asu-miR87b-3p was shown to potentially target IL-33. No matches were found for IL-4 and IL-10.
Table 2. Predicted targets involved in immunity or inflammation of Ascaris suum micro(mi)RNAs in extracellular vesicles (EVs) or supernatant (>100 reads per million) among de-regulated genes in jejunal mucosa of pigs infected with A. suum, relative to uninfected pigs identified by microarray [Citation11]. Abbreviations: A: Adult Excretory/Secretory (ES) EVs; L3: L3 EVs; L4: L4 EVs; ABF: A. suum body fluid; I: Intestine; S: Supernatant.
Proteomic analysis revealed a number of proteins enriched in EVs
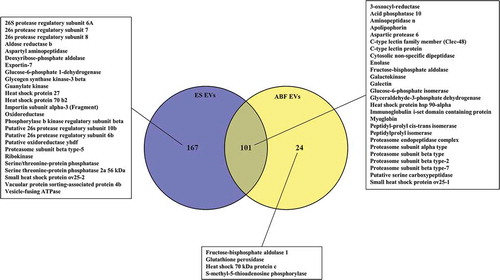
A. suum ES EVs, complete ES-products and EV-depleted ES UC supernatant (ES SN) were analysed using nanoLC-MS/MS in order to examine the protein content. A total of 268, 101 and 129 proteins were identified in the ES EVs, ES and ES SN, respectively (, Supplementary Table 3), where 61 proteins were present in all three samples. A total of 154 proteins were unique for EVs, while only 16 were unique for ES and 5 for ES SN, suggesting a specific repertoire of proteins associated with EVs. Among identified proteins were several proteinases, peptidases, oxidases, reductases, kinases, heat shock proteins and other proteins previously shown to be associated with EVs [Citation32] (see inserted boxes, ). A proteomic analysis was also carried out for ABF EVs, complete ABF and EV-depleted ABF UC supernatant (ABF SN), which revealed a total number of 233, 125 and 198 proteins, respectively (, Supplementary Table 4, Supplementary Table 5). As with ES EVs, the highest number of unique proteins were found in ABF EVs (46 compared to 7 and 39 in ABF and ABF SN, respectively), while 68 were present in all 3 samples (). Hierarchical clustering analysis of the identified proteins showed diverse protein profiles when comparing EVs, ES and SN (Supplementary Figure 2). This analysis shows that EVs are a separate fraction from ES and SN containing specific proteins. The same picture is seen for EVs isolated from ABF (Supplementary Figure 3, Supplementary Table 4, Supplementary Table 5). By comparing ES EVs and ABF EVs () 101 proteins were shown to be shared, leaving only 24 unique ABF EV proteins, compared to 167 for ES EVs, suggesting a higher complexity of the latter. All reported proteins have a false discovery rate (FDR) of <1%.
Figure 6. Venn diagram of proteins associated with Ascaris suum adult Excretory/Secretory (ES) extracellular vesicles (EVs), A. suum adult ES and A. suum adult ES supernatant (SN). A selection of identified proteins are listed (the full list can be found in Supplementary Table 3 (.xls)). Significant in SN, Significant in EV.
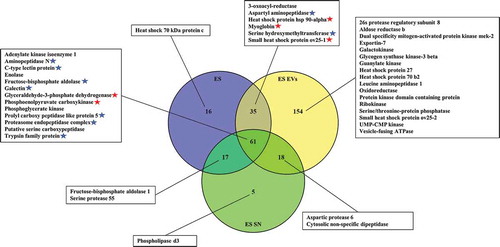
Figure 7. Venn diagram of proteins associated with Ascaris suum adult body fluid (ABF) EVs, ABF and ABF supernatant (SN). A selection of identified proteins are listed (the full list can be found in Supplementary Table 4 (.xls)). Significant in SN, Significant in EV.
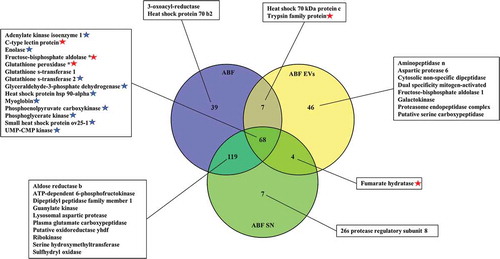
Figure 8. Venn diagram of proteins associated with Ascaris suum adult Excretory/Secretory (ES) extracellular vesicles (EVs) and A. suum adult body fluid (ABF) EVs. A selection of identified proteins are listed (the full list can be found in Supplementary Table 3 (.xls) and Supplementary Table 4 (.xls)).
Discussion
This study provides the first evidence that the porcine roundworm, A. suum, releases EVs in all stages during the development from L3 to L4 to adult worms. A number of miRNAs that may target key immune genes were identified in the EV enriched fraction as well as proteins with immunomodulatory properties suggesting that EVs released by A. suum may play a role in host–parasite interactions.
Comparison of the miRNA composition within EVs of A. suum L3, L4 and adult worms revealed that most of the identified miRNAs were common for the three parasite life stages. However, EVs of L3, which is the stage where the parasite migrates within the host, were the most distinctive as these contained 15 miRNAs not present in EVs released from any of the later developmental stages that reside in the small intestine. It is intriguing to speculate that these miRNAs could play a regulatory role in cellular processes deployed during larval migration, such as tissue proteolysis (and subsequently tissue repair) and evasion of innate host immune responses mounted against the larvae and thereby be important for the initial establishment and survival in the host. It was furthermore shown that the majority of the miRNAs identified in A. suum adult ES EVs and adult ES supernatant were shared. This could indicate that the packing of miRNAs into EVs is rather unspecific and that extracellular miRNAs also is released in a non-EV form, i.e. bound in protein complexes as has been described for extracellular miRNA in plasma and cell cultures [Citation33,Citation34]. Another explanation could be that the EV pellet was contaminated with miRNA-protein complexes from the supernatant. However, the finding of a large number of proteins being unique for EVs compared to the supernatants (–; Supplementary Figure 2–3) suggest that this is not a major problem.
As the release of EVs has not previously been described for Ascaris spp., neither has the origin of released EVs nor the route of EV release. Three potential scenarios are 1) EVs are secreted from intestinal lining and are released through the anal pore, 2) EVs originate from the body fluid and are released through the excretory pore or 3) a combination of the two. We assumed that the similarity of miRNA composition could indicate the route of EV release. In order to examine this, the miRNA composition of EVs purified from intestinal tissue and ABF were compared with the miRNA composition of adult ES EVs (). The majority of identified miRNAs were shared among the three groups and both the origin and excretion route therefore remains unknown. However, our results indicate that both excretion sites contribute, thus released EVs may originate from both the ABF and the intestinal lining. In addition, it cannot be excluded that the ABF contain EVs that have been absorbed from the intestine by coelomocytes [Citation35].
In order to explore a potential functional role of the identified A. suum miRNAs, a target prediction analysis was carried out. As these predictions are known to be associated with high false positive rates [Citation36], we chose to focus on a set of genes that have previously been shown to be significantly up- or down-regulated in jejunal mucosa of A. suum infected pigs compared to uninfected control pigs [Citation13]. Seven of the identified potential target genes, that were down-regulated in infected pigs, encode proteins involved in immunological events such as pathogen recognition, antigen-processing and T-cell co-stimulation (, Supplementary Table 2). In order to identify other potential miRNA targets among genes encoding cytokines involved in helminth immune responses, the full porcine genome was scanned for matches. The analysis revealed that IL-13 mRNA is a potential target for asu-lin-4-5p and asu-let-7-5p, which were identified in all examined samples. That miRNAs of the let-7 miRNA family can target IL-13 has previously been validated and confirmed in vivo in an experimental murine model for asthma [Citation37] and has also been suggested to play a role during infection with the parasitic nematode, Trichuris suis, in pigs [Citation38]. The cytokines IL-25 and IL-33 were furthermore identified as potential targets for A. suum miRNAs (asu-miR-5350d-5p, and asu-miR-87a-3p and asu-miR87b-3p, respectively). Whether these are true targets needs validation and we are aware that many more potential miRNA targets could be present within the porcine genome. Nevertheless, this study suggests that larvae as well as adults worms of A. suum release extracellular miRNAs that potentially can target and regulate porcine genes encoding proteins involved in the host immune response.
The characterization of the A. suum EVs proteome identified several kinases, oxidases, reductases, proteinases and peptidases as has previously been reported to be abundant in parasite EVs [Citation39]. Proteases released by helminths have been suggested to function in metabolic food processing, tissue penetration during migration and in host immunomodulation by degrading immune cell surface receptors and intestinal mucins [Citation40–Citation42]. We therefore suggest that EV-associated proteases may modulate the host immune response against A. suum. In addition, several heat shock proteins (HSPs) were identified, which are also commonly found in EVs [Citation43]. HSPs have been shown to play a key role in the activation of innate immunity, inducing maturation of APCs and providing polypeptides for triggering adaptive immune responses [Citation44]. Among the identified HSPs is HSP70, which has been shown to induce a pro-inflammatory response in both macrophages and DCs [Citation45,Citation46]. In addition, the EVs were shown to contain exportin-7, which is related to exportin-5 (involved in export of pre-miRNAs out of the nucleus [Citation47]), as well as Vesicle-fusing ATPase, which is associated with vesicle-mediated transport [Citation48]. Furthermore, as mentioned, C-type lectins, such as Galectin, were associated with the A. suum EVs, which have shown to be involved in the cellular uptake of EVs [Citation49].
We did not identify tetraspanins in the A. suum EVs, which is in contrast to EVs released by trematodes, where several members of the tetraspanin family have been described [Citation50,Citation51]. However, an absence of tetraspanins has also been shown for Brugia malayi [Citation52] and Teladorsagia circumcincta [Citation53], whereas for N. brasiliensis [Citation54], H. polygyrus [Citation26] and T. muris [Citation39,Citation55], one or two tetraspanins has been reported. The absence or low abundance of tetraspanins in EVs from parasitic nematodes compared to trematodes has been suggested to be related to differences in EV release [Citation39].
EVs purified by use of the IZON qEV size exclusion columns should elute predominantly in fractions 7, 8 and 9, while fractions beyond 10 will contain a higher amount of protein and less EVs [Citation56]. In this study, the presence of particles within the size range of EVs (30–1000 nm) were identified for samples obtained by UC as well as in SEC fractions 7–9. For ABF, no particles were detected in SEC fraction 7, which could be due the rather viscous nature of the ABF, compared to ES, delaying the EV migration through the column. It should, however, also be noted that for both ES and ABF, particles of size corresponding to that of EVs were also detected in SEC fractions 10–12, although at a lower concentration. To confirm whether detected particles in the different fractions are EVs or e.g. contaminating protein aggregates requires further analysis (e.g. flow cytometry) as NTA does not differentiate between these [Citation57]. In general, more size variation was observed for samples purified by SEC compared to UC. Since there is no gold standard for NTA of A. suum EVs it is not possible to specify which size distribution is expected, i.e. one large population of ~100–200 nm or a variety of subpopulations. Therefore, the larger variation observed in samples purified by SEC could reflect that this method better captures different EV subpopulations, but also that the samples contain a larger variety of contaminating protein aggregates and/or EV aggregates. This aspect further highlights the importance of an in-depth characterization of each particle peak.
In summary, this study provides the first evidence of EV release in larvae as well as adults of the large roundworm of pigs, A. suum. Altogether, the results suggest that A. suum EVs and associated molecules may play an important role in the host–parasite interplay. The potential interference with the host immune response could serve as a brilliant survival mechanism of the parasites and may explain why they are able to remain in their host for extensive periods of time without being expelled. Not only does this study provide new fundamental insight to parasite biology, but furthermore opens up for the exploration of parasite EVs and their constituents in diagnosis and infection control. A range of potential applications of EVs exist in this regard, e.g. as biomarkers to detect pre-patent infections and as delivery vehicles for drugs and vaccines. Furthermore, miRNAs that are essential for parasite survival may be applicable as targets in anti-parasitic drugs, serving as alternatives to traditional anthelmintics. Due to the high genetic similarity between A. suum and the human counterpart, A. lumbricoides, this research is likely not only to improve pig welfare but also benefit human health.
Supplemental Material
Download Zip (3.5 MB)Acknowledgements
We thank Sumrin Sahaar for help with worm dissections, Gry Persson for assistance with the ultracentrifuge, Tina V.A. Hansen, Sophie Stolzenbach and Laura J. Myhill for providing parasite material, Sara Almeida and Helene Midttun for help with PBMC purifications. The staff at Servicio Central de Soporte a la Investigación Experimental (SCSIE), Universitat de València are acknowledged for their assistance with the pilot proteomic studies. The Siberian Branch of the Russian Academy of Sciences (SB RAS) Siberian Supercomputer Center is gratefully acknowledged for providing supercomputer facilities.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplementary data for this article can be accessed here
Additional information
Funding
References
- Nansen P, Roepstorff A. Parasitic helminths of the pig: factors influencing transmission and infection levels. Int J Parasitol. 1999;29:877–13.
- Steenhard NR, Jungersen G, Kokotovic B, et al. Ascaris suum infection negatively affects the response to a Mycoplasma hyopneumoniae vaccination and subsequent challenge infection in pigs. Vaccine. 2009;27:5161–5169.
- Pullan RL, Smith JL, Jasrasaria R, et al. Global numbers of infection and disease burden of soil transmitted helminth infections in 2010. Parasit Vectors. 2014;7:1–19.
- Boes J, Helwigh AB. Animal models of intestinal nematode infections of humans. Parasitology. 2000;121:S97–S111.
- Cooper PJ, Figuiredo CA. Immunology of Ascaris and immunomodulation. In: Holland C, editor. Ascaris: the neglected parasite. London (UK): Elsevier; 2013. p. 5–9.
- Hepworth MR, Grencis RK, Artis D. Regulation of innate immunity and inflammation following intestinal helminth infection. Parasit Nematodes Mol Biol Biochem Immunol. 2nd ed. Oxfordshire, UK.: CAB International; 2013. p. 106–122.
- Schopf LR, Hoffmann KF, Cheever AW, et al. IL-10 is critical for host resistance and survival during gastrointestinal helminth infection. J Infect Dis. 2002;168:2383–2392.
- Smallwood TB, Giacomin PR, Loukas A, et al. Helminth immunomodulation in autoimmune disease. Front Immunol. 2017;8:1–15.
- Ferreira AP, Faquim ES, Abrahamsohn IA, et al. Immunization with Ascaris suum extract impairs T cell functions in mice. Cell Immunol. 1995;162:202–210.
- Favoretto B, Casabuono A, Portes-Junior J, et al. High molecular weight components containing N-linked oligosaccharides of Ascaris suum extract inhibit the dendritic cells activation through DC-SIGN and MR. Mol Immunol. 2017;87:33–46.
- van Liempt E, van Vliet SJ, Engering A, et al. Schistosoma mansoni soluble egg antigens are internalized by human dendritic cells through multiple C-type lectins and suppress TLR-induced dendritic cell activation. Mol Immunol. 2007;44:2605–2615.
- Kuijk LM, Klaver EJ, Kooij G, et al. Soluble helminth products suppress clinical signs in murine experimental autoimmune encephalomyelitis and differentially modulate human dendritic cell activation. Mol Immunol. 2012;51:210–218.
- Midttun H, Acevedo N, Skallerup P, et al. Ascaris suum infection down-regulates inflammatory pathways in the pig intestine in vivo and in human dendritic cells in vitro. J Infect Dis. 2017;217:310–319.
- Almeida S, Nejsum P, Williams AR. Modulation of human macrophage activity by Ascaris antigens is dependent on macrophage polarization state. Immunobiology. 2017;223:405–412.
- Andaloussi SEL, Mäger I, Breakefield XO, et al. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov. 2013;12:347–357.
- Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200:373–383.
- Marcilla A, Martin-Jaular L, Trelis M, et al. Extracellular vesicles in parasitic diseases. J Extracell Vesicles. 2014;3(1):25040.
- Szempruch AJ, Dennison L, Kieft R, et al. Sending a message: extracellular vesicles of pathogenic protozoan parasites. Nat Rev Microbiol. 2016;14:669–675.
- Coakley G, McCaskill JL, Borger JG, et al. Extracellular vesicles from a helminth parasite suppress macrophage activation and constitute an effective vaccine for protective immunity. Cell Rep. 2017;19(8):1545–1557.
- Atayde VD, Aslan H, Townsend S, et al. Exosome secretion by the parasitic protozoan Leishmania within the sand fly midgut. Cell Rep. 2015;13:957–967.
- Fromm B, Trelis M, Hackenberg M, et al. The revised microRNA complement of Fasciola hepatica reveals a plethora of overlooked microRNAs and evidence for enrichment of immuno-regulatory microRNAs in extracellular vesicles. Int J Parasitol. 2015;45(11):697–702.
- Gregory RI, Chendrimada TP, Cooch N, et al. Human RISC couples microRNA biogenesis and posttranscriptional gene silencing. Cell. 2005;123(4):631–640.
- Orang AV, Safaralizadeh R, Kazemzadeh-Bavili M. Mechanisms of miRNA-mediated gene regulation from common downregulation to mRNA-specific upregulation. Int J Genomics. 2014;2014:1–15.
- Lindsay MA. microRNAs and the immune response. Curr Opin Pharmacol. 2009;9:514–520.
- Fromm B, Ovchinnikov V, Høye E, et al. On the presence and immunoregulatory functions of extracellular microRNAs in the trematode Fasciola hepatica. Parasite Immunol. 2017;39:e12399.
- Buck AH, Coakley G, Simbari F, et al. Exosomes secreted by nematode parasites transfer small RNAs to mammalian cells and modulate innate immunity. Nat Commun. 2014;5:5488.
- Martin-Jaular L, Nakayasu ES, Ferrer M, et al. Exosomes from Plasmodium yoelii-infected reticulocytes protect mice from lethal infections. PLoS One. 2011;6:1–10.
- Marcilla A, Trelis M, Cortés A, et al. Extracellular vesicles from parasitic helminths contain specific excretory/secretory proteins and are internalized in intestinal host cells. PLoS One. 2012;7(9):e45974.
- Slotved HC, Barnes EH, Bjørn H, et al. Recovery of Oesophagostomum dentatum from pigs by isolation of parasites migrating from large intestinal contents embedded in agar-gel. Vet Parasitol. 1996;63:237–245.
- Théry C, Clayton A, Amigorena S, et al. Isolation and characterization of exosomes from cell culture supernatants. Curr Protoc Cell Biol. New Jersey (USA): John Wiley & Sons, Inc.; 2006. p. 3.22.1–3.22.29.
- Kertesz M, Iovino N, Unnerstall U, et al. The role of site accessibility in microRNA target recognition. Nat Genet. 2007;39(10):1278–1284.
- Kalra H, Simpson RJ, Ji H, et al. Vesiclepedia: a compendium for extracellular vesicles with continuous community annotation. PLoS Biol. 2012;10:8–12.
- Turchinovich A, Weiz L, Langheinz A, et al. Characterization of extracellular circulating microRNA. Nucleic Acids Res. 2011;39:7223–7233.
- Arroyo JD, Chevillet JR, Kroh EM, et al. Argonaute 2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci. 2011;108(12):5003–5008.
- Decraemer W, Coomans A, Baldwin J. Handbook of Zoology, Gastrotricha, Cycloneuralia and Gnathifera. Berlin: De Gruyter;2014 1–60. Chapter1, Morphology of Nematoda.
- Pinzón N, Li B, Martinez L, et al. MicroRNA target prediction programs predict many false positives. Genome Res. 2017;27:234–245.
- Polikepahad S, Knight JM, Naghavi AO, et al. Proinflammatory role for let-7 microRNAs in experimental asthma. J Biol Chem. 2010;285(39):30139–30149.
- Hansen EP, Kringel H, Thamsborg SM, et al. Profiling circulating miRNAs in serum from pigs infected with the porcine whipworm, Trichuris suis. Vet Parasitol. 2016;223:30–33.
- Eichenberger RM, Talukder MH, Field MA, et al. Characterisation of Trichuris muris secreted proteins and extracellular vesicles provides new insights into host-parasite communication. J Extracell Vesicles. 2018;7:1428004.
- Yang Y, Wen YJ, Cai YN, et al. Serine proteases of parasitic helminths. Korean J Parasitol. 2015;53531:1–11.
- Hasnain SZ, McGuckin MA, Grencis RK, et al. Serine protease(s) secreted by the nematode Trichuris muris degrade the mucus barrier. PLoS Negl Trop Dis. 2012;6(10):1–13.
- Nishikado H, Fujimura T, Taka H, et al. Cysteine protease antigens cleave CD123, the α subunit of murine IL-3 receptor, on basophils and suppress IL-3-mediated basophil expansion. Biochem Biophys Res Commun. 2015;460(2):261–266.
- Yáñez-Mó M, Siljander PRM, Andreu Z, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4:1–60.
- Colaco CA, Bailey CR, Walker KB, et al. Heat shock proteins: stimulators of innate and acquired immunity. Biomed Res Int. 2013;2013:1–11.
- Anand PK, Anand E, Bleck CKE, et al. Exosomal Hsp70 induces a pro-inflammatory response to foreign particles including mycobacteria. PLoS One. 2010;5(4):e10136.
- Redzovic A, Gulic T, Laskarin G, et al. Heat-shock proteins 70 induce pro-inflammatory maturation program in decidual CD1a+ dendritic cells. Am J Reprod Immunol. 2015;74:38–53.
- Liu X, Fortin K, Mourelatos Z. MicroRNAs: biogenesis and molecular functions. Brain Pathol. 2008;18:113–121.
- Wang D, Hiesinger PR. The vesicular ATPase: a missing link between acidification and exocytosis. J Cell Biol. 2013;203:171–173.
- Barrès C, Blanc L, Bette-Bobillo P, et al. Galectin-5 is bound onto the surface of rat reticulocyte exosomes and modulates vesicle uptake by macrophages. Blood. 2010;115:696–705.
- Sotillo J, Pearson M, Potriquet J, et al. Extracellular vesicles secreted by Schistosoma mansoni contain protein vaccine candidates. Int J Parasitol. 2016;46(1):1–5.
- Cwiklinski K, De la Torre-Escudero E, Trelis M, et al. The extracellular vesicles of the helminth pathogen, Fasciola hepatica : biogenesis pathways and cargo molecules involved in parasite pathogenesis. Mol Cell Proteomics. 2015;14(12):3258–3273.
- Zamanian M, Fraser LM, Agbedanu PN, et al. Release of small RNA-containing exosome-like vesicles from the human filarial parasite Brugia malayi. PLoS Negl Trop Dis. 2015;9:1–23.
- Tzelos T, Matthews JB, Buck AH, et al. A preliminary proteomic characterisation of extracellular vesicles released by the ovine parasitic nematode, Teladorsagia circumcincta. Vet Parasitol. 2016;221:84–92.
- Eichenberger R, Ryan S, Jones L, et al. Hookworm secreted extracellular vesicles interact with host cells and prevent inducible colitis in mice. Front Immunol. 2018;9:1–14.
- Shears RK, Bancroft AJ, Hughes GW, et al. Extracellular vesicles induce protective immunity against Trichuris muris. Parasite Immunol. 2018;40:e12536.
- IZON. qEV size exclusion column - specifications and operational guide. iZON Science; 2016. p. 1–8. Available from: https://www.schaefer-tec.it/sites/default/files/qEVoriginal%20Technical%20Note%202018.pdf
- Böing AN, Van Der Pol E, Grootemaat AE, et al. Single-step isolation of extracellular vesicles from plasma by size-exclusion chromatography. J Extracell Vesicles. 2014;1;3(1):23430.