ABSTRACT
There is an increasing interest in exploring clinically relevant information that is present in body fluids, and extracellular vesicles (EVs) are intrinsic components of body fluids (“liquid biopsies”). In this report, we will focus on blood. Blood contains not only EVs but also cells, and non-EV particles including lipoproteins. Due to the high concentration of soluble proteins and lipoproteins, blood, plasma and serum have a high viscosity and density, which hampers the concentration, isolation and detection of EVs. Because most if not all studies on EVs are single-centre studies, their clinical relevance remains limited. Therefore, there is an urgent need to improve standardization and reproducibility of EV research. As a first step, the International Society on Extracellular Vesicles organized a biomarker workshop in Birmingham (UK) in November 2017, and during that workshop several working groups were created to focus on a particular body fluid. This report is the first output of the blood EV work group and is based on responses by work group members to a questionnaire in order to discover the contours of a roadmap. From the answers it is clear that most respondents are in favour of evidence-based research, education, quality control procedures, and physical models to improve our understanding and comparison of concentration, isolation and detection methods. Since blood is such a complex body fluid, we assume that the outcome of the survey may also be valuable for exploring body fluids other than blood.
Introduction
At present, there is a growing concern about reproducibility in biomedical sciences [Citation1–Citation4]. This holds true for the new and exponentially growing field of extracellular vesicles (EVs). To improve rigor and reproducibility, minimal requirements, position papers, and guidelines have been published by the International Society for Extracellular Vesicles (ISEV) as well as other societies, and platforms, for example EV-TRACK, were launched [Citation5–Citation8]. Because EVs are a relatively new field of research, we have the unique opportunity to improve the standard of our research by learning lessons from other related fields, which may give us a head start on addressing the problem of reproducibility as encountered elsewhere in biomedical sciences.
Despite these attempts, we are still facing a “Catch 22 problem” wherein we lack reliable analytics, which in turn hampers monitoring pre-analytical variables. For example, even today, with thousands of papers being published on EVs annually, we are still unable to accurately measure all EVs in any given sample. Even for flow cytometry, thus far the most widely used method to detect single EVs, still a 1,000-fold difference is observed in the concentration of EVs in human plasma across different studies [Citation9].
ISEV is working towards improvements for the standardization and reproducibility of EV research to improve the comparability of results between instruments and institutes. The blood EV work group has an important task to improve the rigor and reproducibility of blood collection and handling for studies on EVs, which in turn are prerequisites to establish reliable biorepositories for EV biomarker research. Because blood is a complex fluid and the most studied biofluid for EV biomarker research, likely lessons can be learned here and applied to other body fluids, such as urine, saliva and cerebrospinal fluid. Besides the more general challenges there are also blood-specific challenges to which the blood EV work group will pay attention [Citation10–Citation14].
During the ISEV 2018 meeting in Barcelona, Spain, there were presentations at the inaugural blood EV workgroup by Aled Clayton (AC), Chris Gardiner (CG), An Hendrix (AH), Kenneth Witwer (KW) and Rienk Nieuwland (RN). Based on these presentations and the ISEV Biomarker workshop in 2017, Birmingham, UK, a list of variables was prepared with regard to blood collection, handling and storage (Supplementary Table 1). Then, a brief questionnaire was sent to the kick-off team (to ask their opinion about: (1) Inviting a limited number of (possibly non-ISEV) experts, (2) to go for evidence-based guidelines, and (3) whether to characterize the end-product (in addition to describing the applied methodology), e.g. by measuring the concentration of residual platelets. Answers were unanimously “Yes” to the three questions. The information from this first questionnaire was used to develop a second questionnaire (RN; June 2018), which was sent to and filled in by 22 blood EV work group participants, including 6 members of the ISEV board. Two partipants joined later, and RN did not fill in the questionnaire.
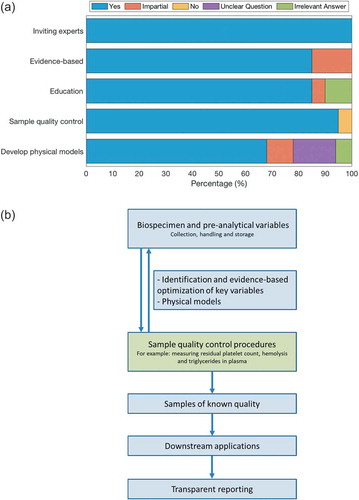
The present report summarizes the outcome of this second questionnaire, which we hope may help to develop a sensible, acceptable and straightforward roadmap to improve the rigor and reproducibility of measurements of EVs in blood. To exclude bias and prejudice, the feedback from all participants have been summarized as quotes in the supplementary information because many answers contain valuable and relevant information or advice. ) summarizes the responses to the most pertinent questions, and highlights a good agreement amongst the participants. In ) the outcomes of this questionnaire are used to develop a straightforward roadmap to improve EV measurements in blood. The results reported here have also been used to build a survey that was launched amongst all ISEV members before the ISEV 2019 meeting in Kyoto, Japan, during which a sneak preview of the results from this survey were shown.
Questionnaire
The questionnaire consisted of questions regarding the background and experience in working with blood and EVs, followed by the specific questions which will be discussed in this report (please see Supplementary information, Questionnaire).
Background of participants
Of the 22 participants, 20 are academic scientists and two are clinician-scientists. Seventeen participants have ≥ 5 years of experience in working with blood samples for EV research.
Involvement of non-ISEV members
All participants (100%) were in favour of involvement of non-ISEV members (Supplementary Table 2; )). To the related question “When inviting non-EV experts (e.g. from more clinical societies), do you think it is important to do this strategically?”, 27% of participants indicated that experts should be selected based on experience, while 64% was in favour of selection based on their strategic position (Supplementary Table 3). In summary, there was consensus that experts from outside ISEV should be invited when deemed necessary or helpful, and these experts should be chosen on experience and/or their strategic position, e.g. regarding their influence within societies, on legislation, etc. Also the involvement of working groups from other societies could be a valuable addition.
Do we go for evidence-based guidelines?
During the ISEV Biomarker workshop, a list of variables associated with blood collection, handling and storage was prepared (Supplementary Table 1), and it was questioned whether recommendations about all variables should be evidence-based. Of the participants, 86% were in favour of evidence-based guidelines, while 14% raised concerns about feasibility (Supplementary Table 4, )). Taken together, most participants are in favour of evidence-based guidelines, at least in part or when feasible. As an alternative, “majority-based” or “consensus-based” instead of “evidence-based” were suggested, but in case of “not evidence-based” this limitation should be made clear in manuscripts, etc.
Should we address all pre-analytics variables, or (only) those considered to be most critical?
Of the participants, 41% was in favour of addressing all variables, two participants (9%) suggested to address all variables to identify the critical steps, while 50% was in favour of identifying the most critical steps. One participant did not answer the question. In summary, 59% support an evidence-based approach to identify the most critical steps (Supplementary Table 5).
How to identify the critical steps?
The participants came up with three suggestions: (1) questionnaire (45%), (2) Rand-type approach (sending a questionnaire to experts; 20%) and (3) literature search (15%). Of note, one participant did not answer the question, one participant was critical about questionnaires, one participant noticed that a questionnaire and Rand approach are likely to give the same end-result, one participant pointed out that a Rand-type approach may be valuable when experts from difference fields/competence are included, and one participant suggested “Consensus – as they are a mean to measure progress in themselves with time and knowledge”. Taken together, in case the critical steps must be identified, one or more possibly combined options are recommended by participants, which include questionnaires, Rand-type approach, and literature survey.
Do critical steps depend on the downstream application?
In total, 17 participants (77%) agreed with this proposition, and most participants provided one or more examples, summarized in Supplementary Table 6.
Is there a role for education?
Most participants (86%) clearly considered education important, although some noticed that it may be too early given the lack of evidence and complexity (), Supplementary Table 7). Alternatively, some participants thought education is useful to raise awareness for standardization and reproducibility. Finally, some participants suggested the involvement of other societies and/or working groups. Some participants pointed out that education must be based on facts, and several suggestions were made as to how education could be set-up.
Quality control of prepared plasma and serum?
Although pre-analytical variables can be recorded in EV-TRACK [Citation5], even a detailed standard operating procedure to prepare for example plasma offers no guarantee that the prepared samples are comparable between individuals and laboratories. Therefore, the participants were asked their opinion on describing the plasma or serum in a quantitative manner. For example, the goal of blood centrifugation protocols is to prepare platelet-depleted plasma. Then why not measure and quantify the residual platelet count in the “platelet-depleted” plasma to monitor the efficacy of the centrifugation procedure, etc.? A quantitative description of the prepared samples provides insight into the quality and consistency of the prepared plasma or serum samples, and enables setting-up reliable biorepositories for future research on EVs. Indeed, 95% of the participants support this approach (), Supplementary Table 8). Two participants already measure residual platelets, and one participant had reservations.
Taken together, there is nearly consensus on describing the end-product, in our case the EV-containing plasma or serum, in a quantitative manner. Which parameters are relevant and should be measured is a matter of debate and specific training and education may be involved, but the goal is to improve (and check) the efficacy of described procedures, and to monitor the quality and consistency of samples prepared for experimental evaluation and biorepositories.
Are physical models a useful instrument to improve our understanding of procedures being used to prepare ev-containing fluids?
Some pre-analytical steps may apply not only to blood but also to other body fluids, e.g. centrifugation protocols. In centrifugation models, density and viscosity of fluids are taken into account, and such models can be used to predict the composition of the end-product, for example with regard to confounders such as residual platelets [Citation15]. Obviously, physical models only have relevance when they can be validated. In that regard, the fluorescence and light scattering models that have been developed to enable the comparison of fluorescence and light scatter signals between flow cytometers are an excellent example of attempts to compare in a quantitative manner the fluorescence, size and concentration of EVs measured on different instruments [Citation16,Citation17].
Although the majority of participants (68%) was in favour of developing such physical models (), Supplementary Table 9), two participants were worried about cross comparison between body fluids (but this is actually precisely where such models may have added value), and in total five answers were discarded (25%): one participant indicated to lack sufficient know-how to answer this question, three answers gave no insight in whether they were in favour or not for the development of physical models, and one participant made a remark about “reference EVs”. The latter is by itself another relevant point, but this was not taken up in the questionnaire in its present form.
Do you think we need multiple road maps (“highways versus byways”)? for example, easy roadmaps for the clinical setting and more detailed roadmaps for laboratories? or per downstream application? other?
An overview of all answers is provided in the Supplementary Table 10. Clearly, some participants are in favour of multiple road maps, some in favour of multiple road maps sharing commonalties, and some in favour of a single roadmap. One participant indicated that the downstream application affects the roadmap rather than the difference between clinical setting and laboratories. There were also several participants in favour of a “highway roadmap” for all. One participant suggested that the “Downstream applications should dictate the roadmap.” Two participants did not answer the question, and one participant indicated not to understand the question. Finally, one participant (JW) came up with an interesting and comprehensive long-term picture and the response is depicted fully in the supplemental table 10.
Conclusions
This report is a first step to improve the rigor and reproducibility of blood EV research, and forms part of the basis of a survey that was launched amongst ISEV members before the ISEV meeting in Kyoto (April 2019). In ) an outline is provided for a roadmap towards collection, handling, and storage of blood needed for EV analysis, in which measuring the sample quality prior to the analysis of EVs, will provide direct insight into the efficacy of the preceding blood collection and handling protocols. Moreover, monitoring the specimen quality and transparent reporting will improve the rigor and ultimate reproducibility of downstream analysis. These suggested changes provide a pragmatic and achievable pathway to accelerate the quality of vesicle biomarker discovery and assay design, and will inform best practice for setting-up reliable biorepositories compatible with vesicle-related readouts.
We hope that the outcome of this questionnaire will steer discussions between members of ISEV and other societies and shape further the roadmap towards reproducible and credible research on blood EVs.
Supplemental Material
Download MS Word (38.1 KB)Acknowledgments
We would like to thank Naomi Buntsma (Amsterdam UMC), Josh Welsh (NIH) and Frank Coumans (Amsterdam UMC) for preparing Figures 1a and 1b.
Supplementary material
Supplemental data for this article can be accessed here.
References
- Glenn Begley C, Ellis LM. Raise standards for preclinical cancer research. Nature. 2012;483:531–5.
- Baker M. Is there a reproducibility crisis? Nature. 2016;533:452–454.
- Baker M. Building better biobanks. Nature. 2012;486:141–146.
- Challenges in irreproducible research. Nature. 2018 special. 2018 October 18. https://www.nature.com/collections/prbfkwmwvz/
- EV-TRACK Consortium, van Deun J, Mestdagh P, et al. EV-TRACK: transparent reporting and centralizing knowledge in extracellular vesicle research. Nat Methods. 2017;14:228–232.
- Lotvall J, Hill AF, Hochberg F, et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International society for extracellular vesicles. J Extracell Vesicles. 2014;3:26913.
- Witwer KW, Soekmadji C, Hill AF, et al. Updating the MISEV minimal requirements for extracellular vesicle studies: building bridges to reproducibility. J Extracell Vesicles. 2017;6:1396823.
- Théry C, Witwer KW, Aikawa E, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International society for extracellular vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7:1535750.
- Gasecka A, Böing AN, Filipiak JK, et al. Platelet extracellular vesicles as biomarkers for arterial thrombosis. Platelets. 2017;28(3):228–234.
- Witwer KW, Buzás E, Bemis LT, et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J Extracell Vesicles. 2013;2:10.3402.
- Coumans FAW, Brisson AR, Buzás EI, et al. Methodological guidelines to study extracellular vesicles. Circ Res. 2017;120:1632–1648.
- Ridger VC, Boulanger CM, Angelillo-Scherrer A, et al. Microvesicles in vascular homeostasis and diseases. Position paper of the European Society of Cardiology (ESC) working group on atherosclerosis and vascular biology. Thromb Haemost. 2017;117(7):1296–1316.
- Lacroix R, Judicone C, Mooberry M, et al. Standardization of pre-analytical variables in plasma microparticle determination: results of the International society on thrombosis and haemostasis SSC collaborative workshop. J Thromb Haemost. 2013;11:1190–1193.
- Lacroix R, Judicone C, Poncelet P, et al. Impact of pre-analytical parameters on the measurement of circulating microparticles: towards standardization of protocol. J Thromb Haemost. 2012;10:437–446.
- Rikkert LG, van der Pol E, van Leeuwen TG, et al. Centrifugation affects the purity of liquid biopsy-based tumor biomarkers. Cytometry Part A. 2018;93A:1207–1212.
- van der Pol E, Sturk A, van Leeuwen T, et al. Standardization of extracellular vesicle measurements by flow cytometry through vesicle diameter approximation. J Thromb Haemost. 2018;16:1236–1245.
- Welsh JA, Horak P, Wilkinson JS, et al. FCMPASS software aids extracellular vesicle light scatter standardisation. Cytometry Part A. 2019. (in press). DOI:10.1002/cyto.a.23782