ABSTRACT
Background and Objective: Ibrutinib has recently been approved in Europe for Waldenström Macroglobulinemia (WM) in symptomatic patients who have received at least one prior therapy, or in first-line treatment for patients unsuitable for chemo-immunotherapy. The aim of the study is to estimate the incremental cost-effectiveness ratio (ICER) of ibrutinib in relapse/refractory WM, compared with the Italian current therapeutic pathways (CTP).
Methods: A Markov model was adapted for Italy considering the National Health System perspective. Input data from literature as well as global trials were used. The percentage use of therapies, and healthcare resources consumption were estimated according to expert panel advice. Drugs ex-factory prices and national tariffs were used for estimating costs. The model had a 15-year time horizon, with a 3.0% discount rate for both clinical and economic data. Deterministic and probabilistic sensitivity analyses were performed to test the results strength.
Results: Ibrutinib resulted in increased Life Years Gained (LYGs) and increased costs compared to CTP, with an ICER of €52,698/LYG. Sensitivity analyses confirmed the results of the BaseCase. Specifically, in the probabilistic analysis, at a willingness to pay threshold of €60,000/LYG ibrutinib was cost-effective in 84% of simulations.
Conclusions: Ibrutinib has demonstrated a positive cost-effectiveness profile in Italy.
Introduction
Waldenström Macroglobulinemia (WM) is a B-cell lymphoproliferative disorder characterized by high levels of immunoglobulin M (IgM [macroglobulin]) in peripheral blood and histological bone marrow with evidence of at least 10% lymphoplasmacytic, associated or not with lymphadenopathy and/or splenomegaly [Citation1–Citation6]. High levels of IgM may be responsible for hyperviscosity syndrome but are generally associated with typical fundoscopic findings [Citation2]. It is a rare disease of the elderly, with a median patients’ age of 65 years and a slight predominance of males over females [Citation2,Citation4,Citation7]. The annual incidence in a European standard population was estimated to be 7.3 and 4.2 per million in males and females, respectively [Citation8]. Although indolent, WM remains incurable and despite the probability of survival for several years, even decades, patients suffer from multiple relapses that adversely affect their quality of life (QoL) and activities of daily living [Citation1,Citation9,Citation10].
The treatment of WM is not standardized, and the choice of therapy is highly personalized, determined by the age, symptoms, comorbidities or preferences of the patient [Citation9,Citation11].
Unlike traditional cytotoxic chemotherapy, which affects both tumor cells and healthy cells, the targeted therapy agent ibrutinib focuses on tumor cells and prevents the kinases from being able to signal this tumor cell growth and division [Citation12]. Ibrutinib is intended for selected hematologic cancers; it is a first-in-class, potent, orally administered drug, that covalently binds to Bruton’s tyrosine kinase (BTK) and inhibits B-cell antigen receptor signalling downstream of BTK [Citation13]. Ibrutinib acts by blocking B-cell antigen receptor signalling, thereby reducing malignant proliferation of B-cells and inducing cell death [Citation13].
Ibrutinib was approved for the first time in 2013 by the US Food and Drug Administration (FDA) and in 2014 by the European Medicine Agency (EMA), with the special status of orphan drug. Ibrutinib was previously indicated, as single agent, for the treatment of adult patients with: relapsed or refractory mantle cell lymphoma (MCL), previously untreated chronic lymphocytic leukaemia (CLL). It was also indicated as single agent or in combination with bendamustine + rituximab in patients with CLL who have received at least one prior therapy [Citation14,Citation15].
In 2015 ibrutinib was approved by FDA and EMA for its use in adults who have received previous treatment for their disease, or in previously untreated patients for whom treatment with chemo-immunotherapy is not suitable [Citation9].
The main study in WM showed that monotherapy with ibrutinib was highly active, associated with durable responses, and safe in pre-treated patients with an overall response rate (ORR) of 90.5%, a two-year progression-free survival (PFS) and overall survival (OS) rates of 69.1% and 95.2%, respectively [Citation16].
The emergence of ibrutinib, and other similar drugs, expands treatment options especially in those diseases that are still missing a standard of care; however, the cost of these is a major concern for healthcare payers [Citation17].
The aim of this study is to estimate, from the Italian National Health System (NHS) perspective, the incremental cost-effectiveness ratio (ICER) of ibrutinib in relapsing/refractory (R/R) WM, compared with the current therapeutic pathways (CTP) applied to WM: fludarabine + cyclophosphamide + rituximab (FCR), bortezomib + rituximab (BOR), rituximab + cyclophosphamide + doxorubicin + vincristine + prednisone (RCHOP), bortezomib + dexamethasone + rituximab (BDR), dexamethasone + rituximab + cyclophosphamide (DRC), and bendamustine/rituximab (BR) [Citation1,Citation18–Citation23].
Material and methods
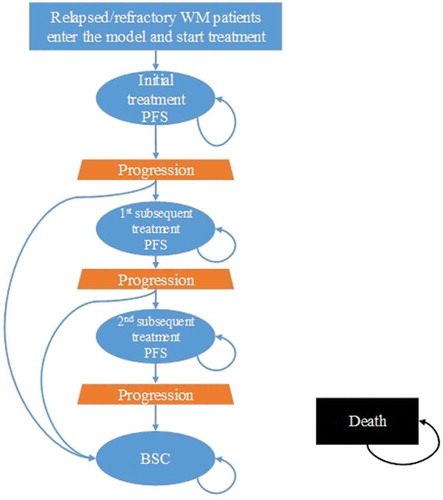
A Markov model (), previously developed to estimate the costs and outcomes associated with WM treatments, was adapted to the Italian healthcare setting.
The model follows patients in a 15-year time-horizon through five health states: initial treatment, first subsequent treatment, second subsequent treatment, best supportive care (BSC),Footnote1 and death. Transition probabilities were used to distribute patients to each health state. Patients enter the model as R/R WM patients in the initial treatment state, and transition out of the health state once progression triggers a treatment change or death occurs. Patients experiencing disease progression will receive subsequent treatment and experience a subsequent progression-free phase, while those refractory to treatment will receive BSC. Patients may be lost due to death during any of the phases.
Costs and health effects are assigned to each health state in a four-week model cycle. As the model progresses cycle-by-cycle for the duration of the time-horizon, costs and utility data are summed per treatment arm, allowing for the calculation of incremental costs and effectiveness per comparator at model completion.
A comparison of patient level data from the 1118e trial and a chart audit (on 452 samples recruited across nine European countries, including Italy, with up to five lines of treatment) were conducted to estimate the efficacy of ibrutinib versus current practice [Citation16]. A cohort of 175 patients was created such that patients had a mix of prior lines of therapy so as to be comparable. Each patient was randomly sampled, so that the mix of therapy lines matched the trial, while the same patient was not allowed to be in two lines.
Clinical input data (efficacy and safety), treatment dosing schedules, overall population mortality were used to populate the model and derived respectively from global trials (2007–2015) as well as published literature [Citation1,Citation16,Citation18–Citation24]. Comparative efficacy for ibrutinib vs. CTP was derived from a multivariate Cox proportional hazard model performed to estimate the hazard ratio (HR) of the PFS for ibrutinib vs. CTP [Citation1,Citation16,Citation18–Citation24]. Missing characteristics data were imputed to ensure sufficient sample size. Given that only three patients died in the ibrutinib trial, it was not feasible to estimate relative treatment effect of ibrutinib on OS. For the estimation of PFS, a Weibull parametric fitting distribution for long-term projection was used.
The percentage use of ibrutinib and other WM therapies, as well as healthcare resources consumption in Italy were estimated according to a panel of clinicians who are experts in the management of WM (). The Italian model assumed a patient with an average weight of 75 kg and a body surface area of 1.8 m2. Health care resources consumption (routine visits and laboratory/instrumental tests, management of adverse events) were costed with both national inpatient and outpatient hospital tariffs while for drugs, ex-factory prices (Euro – €), updated in October 2016, were used [Citation25,Citation26]. The Activity-Based Costing (ABC) methodology was used to estimate the mean yearly cost of the compared patient pathways (–) [Citation27–Citation29]. A 3.0% rate to discount both clinical and economic data was used, as indicated by Italian guidelines [Citation30].
Model outcomes are expressed in terms of incremental costs per life year gained (LYG). We did not consider quality adjusted life years (QALYs), as the experts could not validate the utility values reported in the model.
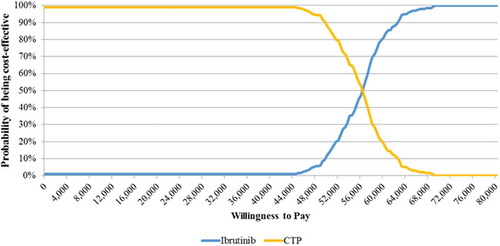
To evaluate the robustness of the model and the results in the Base Case scenario (), both deterministic (DSA) and probabilistic sensitivity analyses (PSA) were performed (; ). In the DSA, parameters were changed through upper and lower bound values (). In the PSA (), where all the variables are changed at the same time, a threshold of €60,000/LYG, in accordance with Italian publications [Citation31], was used to estimate the willingness to pay (WTP) of a healthcare payer for ibrutinib in the treatment of WM.
Table 1. Patients (%) in the cohort analysis treated with different pathways in the CTP group.
Table 2. Drug unit cost.
Table 3. Hospital inpatient unit costs for severe adverse events.
Table 4. Other hospital and outpatient unit costs.
Table 5. Base case analysis.
Table 6. Deterministic sensitivity analysis.
Results
Base case
In the Cox regression, treatment with ibrutinib was the only covariate found to be statistically significant with a HR of 0.25 (95% confidence interval 0.11–0.57; p = 0.001). This is probably due to the relatively small number of progression events and the short follow-up in the trial. The HR for ibrutinib treatment is used in model base case analysis to inform the comparative efficacy of ibrutinib on PFS.
According to literature data [Citation1,Citation16,Citation18–Citation24], the model estimated an incremental value of + 3.0 LYGs vs. CTP (6.77 vs. 3.77) (). On the other hand, as expected, the group treated with ibrutinib showed higher healthcare costs, with an incremental total cost of +€158,198 (€233,066 vs. €74,868) compared to CTP.
The main cost driver, in both arms, was represented by drug cost, with an increment of +€166,626 with ibrutinib vs. CTP. However, such cost was partly offset by a reduction in other healthcare costs such as the management of serious AEs ().
In the base case, the deterministic cost-effectiveness analysis showed a favourable ICER, according to Italian WTP threshold [Citation31] for ibrutinib of €52,698/LYG ().
Deterministic sensitivity analysis
The DSA () demonstrated the strength of the results in the base case. The variation in ibrutinib price (±20% vs. base case) was the main driver in the DSA, with ICERs between €39,343 and €66,054 per LYG. In the majority of the simulations, ICERs are close to the base case results. Particularly when lower HRs were used for the PFS, the ICERs had variations vs. the base case from +1.3% to +3.5%. Apart from the scenarios with a higher price of +20% for ibrutinib and a 10-year time-horizon, all the simulations showed ICERs below a WTP acceptability threshold of €60,000/LYG [Citation31].
Probabilistic sensitivity analysis
The probabilistic sensitivity analysis confirmed the strength of the results. At a WTP threshold of €60,000/LYG [Citation31], ibrutinib was cost-effective in 81% of the simulations () and over a threshold of €68,800/LYG, which can be considered acceptable for a rare disease [Citation32], in 100% of the cases.
Discussion and conclusions
WM is treated most often with rituximab as a monotherapy or in combination with alkylating agents or nucleoside analogues. However, not one of these options is curative and standard of care has not been established [Citation33]. Ibrutinib, a first in-class inhibitor of BTK, displays a unique targeted mechanism of action by inhibiting downstream signalling after the interaction between the mutated MYD88 (Leu265pro) protein, present in more than 90% of patients with WM, and BTK [Citation33–Citation35]. Agents such as rituximab (alone or in combination with bortezomib or bendamustine or fludarabine), do not target disease-specific abnormalities in WM, lack efficacy in WM, and can be associated with serious AEs, particularly in older adults [Citation36]. Given that WM is associated with long survival and generally affects elderly people, aggressively intensifying therapy may not be useful in this population due to potentially life-threatening AEs [Citation37]. The development of second primary malignancies (e.g. myelodysplastic syndrome, acute myeloid leukaemia) from prolonged chemotherapy treatment are, especially for fludarabine based regimens, of particular concern in patients with WM [Citation38,Citation39]. Moreover, the chronic utilization of non-specific developed pharmacological treatment could lead to serious AEs in WM patients with a huge economic impact on healthcare expenditure.
The pivotal, single-arm, phase II trial in previously treated patients with WM who were given ibrutinib had an overall response of 91%, defining ibrutinib as the most active single agent for relapsed or refractory WM to date [Citation16,Citation33].
Given that WM affects few patients, the economic consequences of WM have not been well characterized in the literature. Only a few economic studies were identified while neither cost-effectiveness analyses nor economic evaluations on ibrutinib for WM, published in full, were found [Citation2,Citation40].
Olszewski et al. in 2016 estimated that novel treatments, adopted as a standard of care, may lead to observable changes in both survival and costs of therapy [Citation40]. In the study, mean total Medicare payments for the care of a patient with WM at 15 years from diagnosis were calculated to be $163,432 (€147,301).Footnote2 For the subgroup of patients who received chemotherapy, these costs were nearly twice as high at $193,150 (€174,086)2 compared with those not treated with chemotherapy at $106,705 (€96,173)2. This shift occurred immediately after year 2000 and coincided with widespread rituximab use [Citation40].
A previous Italian study of Annibali et al. in 2005 evaluated only the costs of chemotherapy in patients with WM expressed as cost per unit of surface area for each treatment protocol [Citation2]. In this study of 72 newly-diagnosed patients with WM in Italy, the cost per course of therapy varied from $16/m2 (€14/m2) for oral melphalan/cyclophosphamide/prednisone to $11,091/m2 (€9,996/m2)Footnote2 for cladribine/cyclophosphamide/rituximab. This study did not take into account the medical costs and costs of complications, but only chemotherapy costs [Citation2].
In contrast to previous publications, the cost-effectiveness analysis presented in this paper aimed to describe the total costs and clinical benefits related to the introduction of ibrutinib in the treatment pathways and also the global costs and effects for patients treated with CTP in the Italian setting. Our analysis showed that despite the higher costs of ibrutinib pathway vs. CTP, the ICERs both in the Base Case and in DSA were under the threshold of €60,000/LY indicated as acceptable for Italy, except in the scenario with an increased price of +20% per ibrutinib and a 10-year time-horizon (). However, if we consider an equal value for LYGs and QALYs, due to a lack of evidence on quality of life data for Italian patients, with a WTP threshold for orphan drugs of £50,000/QALY (€68,885/QALY),Footnote3 as reported by Drummond et al. [Citation32], the result is that in every scenario ibrutinib is cost-effective. Also the PSA confirmed the good pharmacoeconomic results of ibrutinib vs. CTP, with a probability of being cost-effective in 81% of the simulations at a WTP threshold of €60,000/LYG ().
Due to the data limitations, the model analysis was subject to a few key uncertainties. The PFS of ibrutinib is projected based on immature Kaplan-Meier data as reported in the 1118e trial; therefore the long-term projection is subject to uncertainty, with a lack of definitive long-term clinical evidence. The mortality of ibrutinib during PFS was assumed to be the same as the general population mortality [Citation24]. Given that only three deaths were reported in the 1118e trial [Citation16], long-term projection of the trial data was not feasible. The mortality during PFS was used to determine the number of patients who would receive subsequent treatments, and consequently drove the post-progression survival. This assumption should be revised when longer follow-up becomes available for ibrutinib-treated patients. On the other hand, it is important to highlight that both the sensitivity analyses stressed the uncertainties of the model and that results are close to the Base Case.
The other relevant assumption is related to the time-horizon. WM is an indolent disease so, according to experts, the scenario with a 20-year time-horizon would be more realistic than the 15-year time-horizon. A longer time-horizon would produce more positive results for ibrutinib (lower ICER); however, we preferred to keep a conservative approach.
The last important issue regarding the cost-effectiveness analysis is the perspective used in the model. If the model had considered the societal perspective, and therefore the indirect costs, ibrutinib would have shown a more positive pharmacoeconomic profile. Ibrutinib has an oral method of administration to the intravenous CTP: patients treated with ibrutinib would need less travelling to the hospital and probably caregivers would incur lower productivity loss to accompany patients needing intravenous CTP or emergency visits due to CTP adverse events.
In conclusion, our study shows that with the use of ibrutinib in patients with WM, less effective palliative therapies and chemotherapy-related AEs can be avoided. In addition, as an oral therapy, ibrutinib avoids the need for infusions and the potential for infusion-related reactions as it can be safely and effectively administered at home. Compared to CTP, the AE profile of ibrutinib also allows it to be safely used in elderly patients and in those with comorbidities that could otherwise limit treatment choice and tolerance [Citation1,Citation16,Citation18–Citation23]. The analysis demonstrates that ibrutinib vs. CTP is a cost-effective therapy in R/R WM patient management and the strength of this positive result was confirmed by the sensitivity analyses which show that in the majority of the simulations the ICERs fall within the WTP threshold of €60,000/LYG.
Ibrutinib is an orphan drug, the use of which contributes towards a significant improvement in the management of patients with WM. This study has also demonstrated that the drug has a positive cost-effectiveness profile in Italy.
Disclosure statement
Aiello A, D’Ausilio A, and Laurenti L report grants from Janssen-Cilag for the conduct of the study. Randon F and Lo Muto R are employees of Janssen-Cilag.
Additional information
Funding
Notes
1 Non-active form of treatment for symptoms management.
2 Conversion rate: €1 = $1.10951; Banca d’Italia. Exchange Rates Archives daily publication and historical series. 2015. Available at https://www.bancaditalia.it/compiti/operazioni-cambi/archivio-cambi/index.html?com.dotmarketing.htmlpage.language=1 Accessed November 2016.
3 Conversion rate: €1 = £0.72585; Banca d’Italia. Exchange Rates Archives daily publication and historical series. 2015. Available at https://www.bancaditalia.it/compiti/operazioni-cambi/archivio-cambi/index.html?com.dotmarketing.htmlpage.language=1 Accessed November 2016.
References
- Tedeschi A, Picardi P, Ferrero S, et al. Bendamustine and rituximab combination is safe and effective as salvage regimen in Waldenström macroglobulinemia. Leuk Lymphoma. 2015;56(9):1–8.
- Annibali O, Petrucci MT, Martini V, et al. Treatment of 72 newly diagnosed Waldenstrom macroglobulinemia cases with oral melphalan, cyclophosphamide, and prednisone: results and cost analysis. Cancer. 2005 Feb 1;103(3):582–587.
- Waldenström JG. Macroglobulinemia–a review. Haematologica. 1986;71:437–441.
- Dimopoulos MA, Alexanian R. Waldenstrom’s macroglobulinemia. Blood. 1994;83:1452–1459.
- Ghobrial IM, Gertz MA, Fonseca R. Waldenström macroglobulinaemia. Lancet Oncol. 2003;4:679–685.
- Dimopoulos MA, Panayiotidis P, Moulopoulos LA, et al. Waldenström’s macroglobulinemia: clinical features, complications, and management. J Clin Oncol. 2000;18:214–226.
- International Waldenström Macroglobulinema Foundation. Information on the disease. Available from: https://www.iwmf.com/
- Buske C, Leblond V, Dimopoulos M, et al.; ESMO guidelines Working Group. Waldenstrom’s macroglobulinaemia: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24:vi155–vi59.
- Castillo JJ, Palomba ML, Advani R, et al. Ibrutinib in Waldenström macroglobulinemia: latest evidence and clinical experience. Ther Adv Hematol. 2016;7:179–186.
- Castillo JJ, Olszewski AJ, Kanan S, et al. Overall survival and competing risks of death in patients with Waldenström macroglobulinaemia: an analysis of the surveillance, epidemiology and end results database. Br J Haematol. 2015;169:81–89.
- Chakraborty R, Kapoor P, Ansell SM, et al. Ibrutinib for the treatment of Waldenström macroglobulinemia. Expert Rev Hematol. 2015 Oct;8(5):569–579.
- Wujcik D. Targeted therapy. In: Yarbro CH, Wujcik D, Gobel BH, editors. Cancer nursing: principles and practice. Sudbury (MA): Jones and Bartlett; 2011. p. 561–583.
- Cameron F, Sanford M. Ibrutinib: first global approval. Drugs. 2014 Feb;74(2):263–271.
- U.S. Food and Drug Administration, Center for Drug Evaluation and Research. Imbruvica® (ibrutinib) capsules, for oral use: highlights of prescribing information. Revised 01/2015. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/205552s002lbl.pdf
- European Medicine Agency. Imbruvica® (ibrutinib). Product information. Product information Imbruvica -EMEA/H/C/003791 -II/0025. [ cited 2017 Mar 31]. Retrieved from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/003791/human_med_001801.jsp&mid=WC0b01ac058001d124
- Treon SP, Tripsas CK, Meid K, et al. Ibrutinib in previously treated Waldenström’s macroglobulinemia. Engl J Med. 2015;372:1430–1440.
- Kastritis E, Dimopoulos MA. Current therapy guidelines for Waldenstrom’s macroglobulinaemia. Best Pract Res Clin Haematol. 2016;29(2):194–205.
- Tedeschi A, Ricci F, Goldaniga MC, et al. Cyclophosphamide, and rituximab in salvage therapy of Waldenström’s macroglobulinemia. Clin Lymphoma Myeloma Leuk. 2013;13:231–234.
- Ghobrial IM, Xie W, Padmanabhan S, et al. Phase II trial of weekly bortezomib in combination with rituximab in untreated patients with Waldenström Macroglobulinemia. Am J Hematol. 2010;85:670–674.
- Buske C, Hoster E, Dreyling M, et al.; German Low-Grade Lymphoma Study Group. The addition of rituximab to front-line therapy with CHOP (R-CHOP) results in a higher response rate and longer time to treatment failure in patients with lymphoplasmacytic lymphoma: results of a randomized trial of the German Low-Grade Lymphoma Study Group (GLSG). Leukemia. 2009;23(1):153–161. https://www.nature.com/leu/journal/v23/n1/full/leu2008261a.html
- Dimopoulos MA, García-Sanz R, Gavriatopoulou M, et al. Primary therapy of Waldenstrom macroglobulinemia (WM) with weekly bortezomib, low-dose dexamethasone, and rituximab (BDR): long-term results of a phase 2 study of the European Myeloma Network (EMN). Blood. 2013;122:3276–3282. 10.1182/blood-2013-05-503862
- Dimopoulos MA, Anagnostopoulos A, Kyrtsonis MC, et al. Primary treatment of Waldenström macroglobulinemia with dexamethasone, rituximab, and cyclophosphamide. J Clin Oncol. 2007;25:3344–3349. 10.1200/JCO.2007.10.9926
- Rummel MJ1, Niederle N, Maschmeyer G, et al. Study group indolent Lymphomas (StiL).Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet. 2013;381(9873):1203–1210.
- ISTAT. Tavole di Mortalità; 2016. Available from: www.istat.it
- Ministero Salute. Remunerazione prestazioni di assistenza ospedaliera per acuti, assistenza ospedaliera di riabilitazione e di lungodegenza post acuzie e di assistenza specialistica ambulatoriale. Decreto 18-Oct-2012. Available from: http://www.salute.gov.it/portale/temi/p2_6.jsp?id=3662&area=programmazioneSanitariaLea&menu=vuoto
- Farmadati. Compendio farmaceutico. Available from: www.farmadati.it
- Baker JJ. Activity-based costing and activity-based management for healthcare. Gaithersburg (MD): Apen; 1998.
- Roffia P. Il controllo di gestione activity-based. Torino: Giappichelli; 2002.
- Innes J, Mitchell F. Activity-based cost management. London: CIMA; 1991.
- Fattore G. Proposta di linee guida per la valutazione economica degli interventi sanitari in Italia. Pharmacoeconomics Ital Res Articles. 2009;11:83–93.
- Messori A, Santarlasci B, Trippoli S, et al. Controvalore economico del farmaco e beneficio clinico: stato dell’arte della metodologia e applicazione di un algoritmo farmacoeconomico. Pharmacoeconomics-Ital Res Articles. 2003;5(2):53–67.
- Drummond MF, Wilson DA, Kanavos P, et al. Assessing the economic challenges posed by orphan drugs. Int J Technol Assess Health Care. 2007;23(1):36–42.
- Dimopoulos MA, Trotman J, Tedeschi A, et al. iNNOVATE study group and the European consortium for Waldenström’s Macroglobulinemia. Ibrutinib for patients with rituximab-refractory Waldenström’s macroglobulinaemia (iNNOVATE): an open-label substudy of an international, multicentre, phase 3 trial. Lancet Oncol. 2017;18(2):241–250.
- Treon SP, Yang G, Zhou Y, et al. MYD88 L265P somatic mutation in Waldenström’s macroglobulinemia. N Engl J Med. 2012 Aug 30;367(9):826–833. DOI:10.1056/NEJMoa1200710
- Yang G, Zhou Y, Liu X, et al. A mutation in MYD88 (L265P) supports the survival of lymphoplasmacytic cells by activation of Bruton tyrosine kinase in Waldenström macroglobulinemia. Blood. 2013;122(7):1222–1232.
- Treon SP, Branagan AR, Hunter Z, et al. Paradoxical increases in serum IgM and viscosity levels following rituximab in Waldenstrom’s macroglobulinemia. Ann Oncol. 2004;15(10):1481–1483. 10.1093/annonc/mdh403
- Gertz M. Waldenström macroglobulinemia: my way. Leuk Lymphoma. 2013;54(3):464–471.
- Leleu X, Roccaro AM, Moreau AS, et al. Waldenstrom macroglobulinemia. Cancer lett. 2008;270(1):95–107.
- Garcia-Sanz R, Ocio EM. Novel treatment regimens for Waldenström’s macroglobulinemia. Expert Rev Hematol. 2010;3(3):339–350.
- Olszewski AJ, Treon SP, Castillo JJ. Evolution of Management and Outcomes in Waldenström Macroglobulinemia: A Population-Based Analysis. Oncologist. 2016 Jul 29. pii: theoncologist.2016-0126. [Epub ahead of print] http://theoncologist.alphamedpress.org/content/21/11/1377