ABSTRACT
Background: Market access stakeholders consider the adoption of Managed Entry Agreements (MEAs), however a clearly described methodology to quantify their implementation burden is not available in the public domain.
Objective: To quantify the cost of implementing a performance-based MEA at the hospital level.
Methods: The analysis involved a hypothetical one-off therapy targeting Acute Lymphoblastic Leukaemia. Data collection from five NHS Hospital Trusts in England captured costs by task, job band, personnel time and capital investment. We compared the administrative burden of the standard of care (SoC) to that of adopting the therapy with or without an MEA over 10 years.
Findings: The 10-year cost for the activities required to support hospital payments for the target patient population in England varied as follows: for the SoC was £447,353, compared to £1,117,024 for the novel therapy with MEA, and £245,317 without MEA.
Conclusions: The higher cost associated with the SoC compared to the novel therapy without an MEA, arises from the higher frequency of infusions requiring payments and the associated mandatory data capturing requirements for oncology therapies. The novel therapy with MEA presents the greatest burden due to increased frequency of monitoring in year one to compensate for the greater uncertainty in clinical data and to inform the performance-based reimbursement.
Introduction and objectives
Regenerative medicines and National Institute for Health and Care Excellence (NICE) appraisals
Regenerative medicines and Advanced Therapy Medicinal Products (ATMPs), such as Chimeric Antigen Receptor T-cell (CAR T-cell) therapies, are a group of treatments that seek to restore the normal function of human organs, tissues or cells. Within this therapy group, adoptive cell transfer (ACT) has been identified as a new but rapidly emerging approach [Citation1,Citation2]. ACT involves collecting T-cells from the patient using apheresis and then programming them to distinguish cancerous from non-cancerous cells. These modified T-cells are infused back into the patient to begin attacking the cancer cells. There are several types of ACT [Citation3], but currently, the one that has advanced furthest in terms of clinical development (and regulatory approval) is a CAR T-cell therapy, which involves adding the specific chimeric antigen receptor to the T-cells [Citation4].
Such therapies may come to market with immature data sets and at a considerable cost, which presents payers with data uncertainty and potential affordability issues. The use of Managed Entry Agreements (MEAs) is increasingly considered as an option to enable adoption and patient access. The promise for the development of future treatments is high, increasing the importance of overcoming barriers and enabling innovation.
In response to the 2013 House of Lords’ inquiry into regenerative medicines, a special National Institute for Health and Care Excellence (NICE) study and Expert Panel were set up, which included the Cell and Gene Therapy Catapult and The Centre for Health Economics (CHE) Technology Assessment Group (University of York), to review a hypothetical CAR T-cell therapy in relapsed (two relapses or more) or refractory B-Acute Lymphoblastic Leukaemia (rB-ALL) within the context of a NICE Technology Appraisal (TA) framework [Citation5]. Different hypothetical evidence sets in terms of data maturity were assessed; it was concluded that performance-based reimbursement mechanisms would go further in supporting a favourable HTA decision where there is a combination of great uncertainty (due to e.g., less mature data sets), and a potentially substantial patient benefit.
Therefore, the evaluation recommended that innovative payment mechanisms for managing and sharing financial risk such as performance-based MEAs should be developed [Citation5].
Managed entry agreements
MEAs are mechanisms by which pharmaceutical companies and payers share financial and clinical risk associated with the introduction of new medicines, and can be classified as either financial (discount), performance-based (outcome-based, value-based and/or linked to conditional terms i.e. collection of evidence), or innovative (novel combination of factors).
The CHE NICE report demonstrated that an outcomes-based staged payment approach over the period of time that benefits (i.e. remission and therefore survival) are sustained, reduces payer decision uncertainty compared to a fixed acquisition cost, whilst providing an exit strategy for the National Health Service (NHS) if the purported clinical benefits did not materialise [Citation5].
Research objectives
To examine and communicate the predicted administrative burden associated with introducing a performance-based MEA using a hypothetical therapy exemplar
To provide a methodological framework for pharmaceutical companies and health systems to utilise in order to explore the cost associated with setting up and implementing a value-based MEA, such as an outcomes-based staged payment approach over the period of time that therapy benefits are sustained (we apply a 10-year horizon in our analysis). It was also intended that the data collection process would be applicable to a range of different MEAs and a variety of therapy areas.
Hypothetical CAR T-cell therapy exemplar
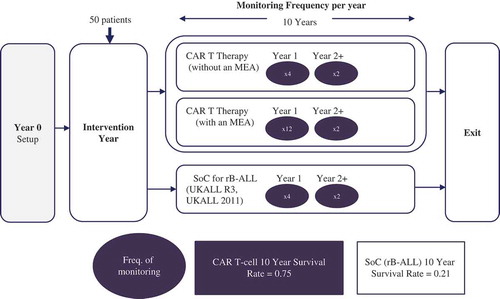
The hypothetical scenario used in this investigation was a CAR T-cell therapy for rB-ALL in children and young adults, compared with the current standard of care (SoC), chemotherapy (UKALL R3, UKALL 2011) [Citation6,Citation7]. We quantified the incremental direct and indirect administrative burden of an MEA at the local NHS Trust level in England. The forecasted cost of this administrative burden was quantified over a 10-year period.
The treatment arm for the CAR T-cell therapy with an MEA reflects a situation where there is greater uncertainty associated with the efficacy and safety data provided to NHS decision-makers at launch (as compared to CAR T-cell therapy without an MEA arm), and this results in a requirement for a higher monitoring frequency in year one.
Reimbursement routes
There are several potential reimbursement routes available () [Citation8–Citation13], but at the time of undertaking this research, no specific route has been selected for any CAR T-cell therapy in England.
Table 1. Reimbursement routes.
Materials and methods
Study setting and sample population
NHS Trusts are responsible for operationalising an MEA; the segmentation criteria () identified 24 suitable NHS Trusts that were targeted. Research participants (chief pharmacists, haematology pharmacists or oncology pharmacists) from five suitably profiled trusts were recruited. Consent forms were signed by these individuals, allowing for their data contributions to be used on an anonymous, aggregated basis.
Table 2. Segmentation criteria.
Study design
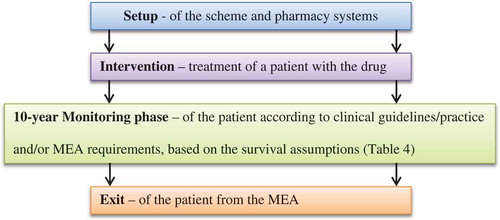
The study was designed with the input of a Project Advisory Group (PAG) consisting of personnel from senior NHS pharmacy and clinical oncology roles, as well as NICE. The full patient pathways () were constructed in consultation with the PAG. A data collection template was designed to capture the time and resources involved in operationalising each of the three pathways.
Qualitative and quantitative data were gathered through an electronic, structured data collection process using Excel and a follow-up, one-to-one, semi-structured telephone interview to explore the data. The Excel file captured the direct and indirect resources by task, job band, personnel time required to complete the task and capital investment.
Costing and survival model assumptions
Direct and indirect cost
The study examined direct and indirect costs (as defined in ) associated with an MEA, excluding the cost of the therapy and associated patient management.
Table 3. Direct and indirect costs.
PAS and survival model assumptions
Key assumptions around the PAS and patient survival are detailed in .
Table 4. Assumptions made using PAG and participant advice.
Based on these assumptions (), MEA payments are made at individual patient-level to the manufacturer over a period of up to 10 years if remission is maintained. Patient survival is used as a proxy for remission in our example, and relapse is assumed to be followed by imminent death (reflecting the severe stage of these patients’ ALL).
Data analysis
Following completion of the research questionnaire by participants, data was entered into a central database and a series of quality assurance tests were applied ().
Table 5. Quality assurance tests.
Clarification of erroneous entries with the participant was undertaken before a clean database of raw data was finalised. Categorisation by personnel activity and capital investment was performed before grouping by hospital department i.e. Pharmacy, Clinical, Finance, HR and Training, Information Technology (IT) and Other.
Salary data was taken from the latest NHS Employers Agenda for Change Pay Scales [Citation14] and multiplication of the mid-point salary with the time taken to perform a task provided a unit cost per activity. These calculated costs were then assigned to the respective stage () of the patient pathway for each of the three treatment arms.
Each arm is an aggregation of all costs for all stages associated with that treatment and MEA, where applicable. Data was analysed by direct versus indirect cost, cost by each of the four stages and by total cost for the treatment arm. The outputs allow for the calculation of the incremental cost of CAR T-cell therapy with an MEA against CAR T-cell therapy without an MEA or existing SoC.
Results
Key findings
All findings are specific to personnel activity and expense associated with the administration and on-going monitoring of a treatment for rB-ALL. The data do not relate to the cost of the therapy or associated patient management.
Total cost
The estimated total 10-year costs to an English NHS Trust (for 50 new patients treated per annum), subcategorised by treatment stage, as measured in pounds (£), are detailed in .
Table 6. 10-year total estimated administrative burden (£) of CAR-T cell therapy, with and without an MEA, and SoC (rB-ALL) split by treatment stage.
The resulting treatment stage-specific incremental costs of adopting the CAR T-cell therapy with an MEA (as compared to the SoC) is £2,594, -£114,147, £738,283 and £15,941 for the Setup, Intervention, Monitoring and Exit stages respectively. It is also worth noting that when comparing the CAR-T cell with and without MEA, the increase in Year 1 monitoring costs (from £150,024 to £572,904) is not linear to the increase in the frequency of monitoring visits (four vs. 12). This is because the CAR-T cell therapy with MEA incurs greater costs per monitoring visit, due to the greater involvement of pharmacy and administrative staff operationalising the MEA.
Furthermore, it should be noted here that if there are multiple national treatment centres adopting the CAR T-cell therapy with an MEA, the additional £2,594 set up costs per hospital will have to be repeated for each new centre. E.g., for three centres this will triple the MEA setup costs to £7,782. However, the national incidence of 50 new patients per year will be treated across three hospitals (instead of one), effectively with each centre treating 16–17 patients. This could result in spreading the remaining £868,484 of cost for the 10-years across the three centres, reducing each hospital trust’s total incremental burden to £289,495.
An estimated total cost, as measured in pounds (£), for the direct and indirect activity associated with SoC, CAR T-cell therapy without an MEA and CAR T-cell therapy with an MEA can be found in .
Table 7. Estimated total direct and indirect activity costs over 10 years.
Incremental cost (direct and indirect) of CAR T-cell therapy with an MEA
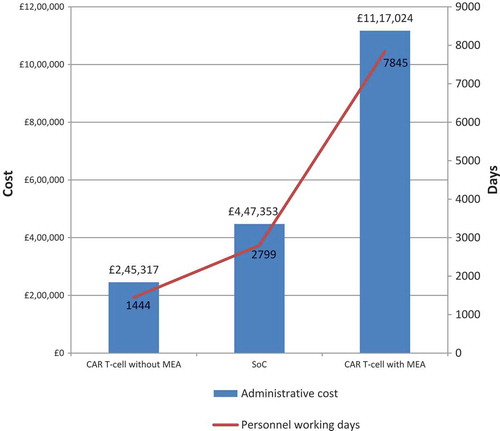
The estimated 10-year incremental cost to an English NHS Trust associated with implementing an MEA for the hypothetical CAR T-cell therapy (for 50 new patients per annum) is displayed in , where the cost differential compared with a hypothetical CAR T-cell therapy without an MEA is £871,707, and compared to the SoC it is £669,671.
Total personnel days (direct and indirect)
The total number of personnel working days used by an English NHS Trust to undertake the administration for 50 new patients per annum treated for rB-ALL over a 10-year period, is estimated at 1,444 working days for hypothetical CAR T-cell therapy without an MEA, 7,845 working days for hypothetical CAR T-cell therapy with an MEA, and 2,799 working days for the existing SoC.
Forecasted incremental burden per patient of CAR T-cell therapy with an MEA
details the estimated 10-year incremental burden per patient to an English NHS Trust associated with implementing an MEA for the hypothetical CAR T-cell therapy.
Table 8. Estimated incremental burden to an English NHS Trust associated with implementing an MEA for CAR T-cell therapy for 50 new patients per annum.
Discussion
Payers and Health Technology Assessment (HTA) bodies want to understand the cost of implementing outcomes-based, innovative contracting payment schemes. This research outlines a qualitative and quantitative assessment of the administrative burden of such a scheme for a hypothetical CAR T-cell therapy in rB-ALL, and provides a methodological approach that is applicable for complex performance-based MEA schemes regardless of therapy arena and indication. We have not been able to identify efforts that go to the same lengths in terms of quantification of the administrative burden of MEAs in the literature from other countries, which highlighting the timeliness of our research.
Incremental burden associated with an MEA for CAR T-cell therapy
The predicted incremental administrative burden of adopting the CAR T-cell therapy with an MEA, compared with a CAR T-cell therapy without an MEA, was found to be £871,707. This equates to an additional 6,401 NHS staff days over the 10-year period modelled (treating 50 new patients per year). This burden reflects a 4.6-time increase in resource use compared to adopting the CAR T-cell therapy without an MEA, borne in part during the set-up and treatment phases, but mainly during the 10-year monitoring phase. The incremental burden of CAR T-cell therapy with an MEA compared with current SoC was £669,671. This represents more accurately the incremental burden existing hospitals delivering SoC today would need to fund if implementing CAR T-cell therapy with an MEA tomorrow (this equates to an extra 5,046 staffing days over the 10-year period).
It is worth noting that the SoC in rB-ALL represents a considerable baseline administrative burden, which arises from A) the requirement for oncology treatments to enter data into the Systemic Anti-Cancer Treatment (SACT) database, as well as BlueTeq for Payment by Results-excluded (PbRe) products, whether an MEA exists or not, and B) the repeated activity for the payment of numerous chemotherapy administrations; this baseline administrative burden for the SoC has been quantified for the first time in this paper.
The forecasted administrative burden associated with the CAR T-cell therapy without an MEA is less resource-intensive than the SoC because it only requires a single upfront payment upon one-off administration.
The CAR T-cell therapy with an MEA presents the greatest burden of the three options analysed, and a key driver for this is the increased frequency of Minimal Residual Disease (MRD) blood testing in year one (due to greater uncertainty around the safety and efficacy data; this also informs the performance-based reimbursement mechanism), but also greater patient numbers over time (as compared to the SoC) due to improved survival.
Resource planning implications
Participants involved in this research recognised the lack of existing quantitative analyses of the administrative burden associated with current MEA/access schemes, and highlighted this as an obstacle to effective resource planning and service provision. This research enables NHS organisations to plan for complex performance-based reimbursement schemes over a prolonged duration. CAR T-cell therapy with an MEA is forecast to require 11 or 15 additional staff working days per patient over 10 years when compared to SoC and CAR T-cell therapy without an MEA, respectively (see ). Further investigation showed that the increase in resource costs is mainly attributable to the increase in pharmacy personnel time (which attributed for 56% of the additional personnel time) and higher banding of these personnel involved (95% of pharmacy activity required senior staff i.e. band 8a or above) and that the indirect costs, e.g., set up costs and infrastructure that would be required for the system to run, are negligible (less than 1%), as the data collection infrastructure is already in place for oncology therapies (through SACT and BlueTeq). From an NHS perspective, this also means that the incremental cost of adopting the CAR T-cell therapy with an MEA at multiple vs. few hospitals would be low.
With NHS hospital staff resources being already overstretched in a constrained fiscal environment, the failure to deploy and plan staff resource appropriately may either lead to the failure of the system to successfully adopt new therapies with such MEA schemes or take staff away from direct front-line patient care duties, neither of which is desirable. HTA bodies and the NHS should aim to quantify this incremental burden to inform MEA endorsement, as well as to facilitate effective future budgeting and planning and ultimately success of the scheme.
Broader implications
As outlined in the University of York paper [Citation5], a CAR T-cell therapy with immature evidence base, and a hypothetical list price of several hundred thousand pounds may present the NHS with a sizeable uncertainty in terms of being cost-effective, as well as affordability issues. An MEA using a 10-year performance-based staged payment approach (i.e. pay for remission only and stop paying for relapsed patients) is a potential means to address both the uncertainty around the sustainability of the patient benefit (in the absence of long-term clinical data) and the affordability issue arising from the NHS having to pay in full upfront. While this solution makes sense intuitively, it comes at a cost, as we have detailed above. Although this cost in absolute terms is considerable, it is relatively modest in comparison to the cost of a product that may have a list price in the hundreds of thousands of pounds per patient. This could potentially also be considered an acceptable ‘annual insurance premium’ to underwrite reimbursement should the treatment not produce the benefits it claims to deliver.
It should be noted that the costs communicated in this study are based on treating 50 patients per annum and over a 10-year horizon and leveraging the existing oncology data infrastructure for enabling the operation of the MEA. However, if a similar MEA arrangement was to be implemented for a larger therapy area in terms of target patient population, then the total administrative burden and associated cost would increase proportionately to the number of patients treated. Similarly, if the target therapy area lacks an existing data collection infrastructure, the total MEA implementation burden would further increase due to the capital investment required to create the appropriate infrastructure for the operation of the MEA. Having a common, established data collection infrastructure such as the one operated in Italy by the Italian Medicines Agency (AIFA) would lower the marginal cost of setting up each new MEA, as well as the barriers for the implementation of performance-based MEAs, and ultimately the hurdles for adopting innovative therapies. We believe that there is a case to be made for joint government and industry investment to create such infrastructure.
It is also important to note that the monitoring requirements for the purpose of an MEA should have a clinical justification as a basis so that patients do not undergo any clinically unnecessary investigations, solely for the purpose of a reimbursement mechanism.
In our example, the CAR T-cell therapy with an MEA reflects a situation where there is considerable uncertainty associated with the clinical data provided at launch and that this is the key driver of the requirement for a higher monitoring frequency in year one. Therefore, one can argue that these incremental costs may not necessarily be solely attributable to the operation of the reimbursement scheme, but that they also address clinical and regulatory considerations around real-world safety and effectiveness.
Finally, it is important to highlight that the incremental costs of introducing a performance-based MEA is likely lower in the oncology area than in other therapy areas, due to the existing SACT/Blueteq data collection infrastructure that is already in use for patients on the SoC. This goes a long way in explaining our finding that the indirect costs (e.g., IT infrastructure) are negligible, and NHS decision-makers need to be mindful that this would not be the case in therapy areas where the data collection infrastructure needs to be established. Establishing a national registry for higher-uncertainty therapies that links clinical outcomes to reimbursement (across different therapy areas), like the one operated by AIFA in Italy, would be one way of reducing the indirect costs in other indications where the infrastructure to adopt MEAs is lacking.
Despite market access stakeholders (HTA bodies, national, regional and local level payers) currently considering whether to adopt MEAs, the additional administrative burden of implementation is often not quantified, thereby limiting their assessment and the future planning of resources. Furthermore, clear guidance on how such burden is quantified is not available to date in the public domain. HTA bodies and the NHS should aim to quantify the incremental burden associated with introducing performance-based MEAs, as well as the dynamics of the relationship between capital investment and marginal cost per patient, in order to make better-informed decisions around their potential endorsement, as well as to facilitate effective future budgeting. We recommend that a methodological approach such as the one exemplified within this research is used by HTA bodies and payer organisations to qualify and quantify resource implications of implementing complex MEAs, as well as by manufacturers in preparation for making such MEA proposals to payers.
During our research, we encountered desire by national strategic bodies such as NHS England (NHSE), Department of Health (DH), and NICE to also quantify their administrative burden when assessing proposals for MEAs; therefore, we propose that the burden of MEA assessments is also quantified so that payer organisations can plan accordingly and enable timely MEA assessments.
Limitations
The study authors acknowledge the following limitations:
1. Although the hospital Trust sample size is small, the sample size will encompass most specialist centres for ALL in England which would be eligible for using CAR T-cell therapy for ALL.
2. At the time of writing this document, CAR-T-cell therapy treatment is not available within the NHS so respondents drew on previous experience when operating PASs, based on a hypothetical therapy profile.
3. The data analysis is linear over a 10-year period and does not consider:
Inflation; Consumer Price Index (CPI)
Employment on-costs such as National Insurance and pension contributions.
Actual survival rates per year, over a 10-year period
4. The rB-ALL arm for the treatment pathway is diverse and exhibits multiple arms, for example with or without Hematopoietic Stem Cell Transplantation (HSCT). The rB-ALL care pathway arm was therefore simplified.
5. The frequency of the MRD blood test for SoC and CAR T-cell therapy was not identified during the literature review. Therefore, blood test frequency within the model was set using ‘clinical judgement’ in conjunction with expert advice.
Acknowledgements
Editorial support was provided by Adam Buckler
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Cancer Research UK. Adoptive cell transfer. 2017 [cited 2018 Jul 27]. Available from: https://www.cancerresearchuk.org/about-cancer/cancer-in-general/treatment/immunotherapy/types/adoptive-cell-transfer
- National Cancer Institute. CAR T cells: engineering patients’ immune cells to treat their cancers. 2017 [cited 2018 Jul 27]. Available from: https://www.cancer.gov/about-cancer/treatment/research/car-t-cells
- Melanoma Research Alliance. Adoptive cell transfer therapy. 2017 [cited 2018 Jul 27]. Available from: https://www.curemelanoma.org/patient-eng/melanoma-treatment/therapies-in-development/adoptive-cell-transfer-therapy/
- American Cancer Society. Chimeric antigen receptor (CAR) T-cell therapy. 2017 [cited 2018 Jul 27]. Available from: https://www.cancer.org/treatment/treatments-and-side-effects/treatment-types/immunotherapy/car-t-cell1.html.
- Hettle, R., et al. The assessment and appraisal of regenerative medicines and cell therapy products: an exploration of methods for review, economic evaluation and appraisal. Health Technol Assess. 2017;21(7):1–8.
- Saha V ALLR3: an international collaborative trial for relapsed and refractory Acute Lymphoblastic Leukaemia (ALL). 2007 [cited 2018 Jul 27]. Available from: http://journals.plos.org/plosone/article/file?type=supplementary&id=info:doi/10.1371/journal.pone.0108107.s003
- Lymphoma Action. UKALL 2011: a phase 3 trial looking at reducing side effects by changing the standard treatment for acute lymphoblastic leukaemia or lymphoblastic lymphoma in children and young people aged 1-24. [cited 2018 Jul 27]. Available from: https://lymphoma-action.org.uk/index.php/trial/ukall-2011-phase-3-trial-looking-reducing-side-effects-changing-standard-treatment-acute
- National institute for Health and Care Excellence (NICE). Technology appraisal guidance. 2018 [cited 2018 Jul 27]. Available from: https://www.nice.org.uk/about/what-we-do/our-programmes/nice-guidance/nice-technology-appraisal-guidance
- Pauwels, K., et al. Managed entry agreements for oncology drugs: lessons from the European experience to inform the future. Front Pharmacol. 2017;8(171).
- van de Vooren, K., et al. Market-access agreements for anti-cancer drugs. J R Soc Med. 2015;108(5):166–170.
- National institute for Health and Care Excellence (NICE). Patient access schemes liaison unit. 2018 [cited 2018 Jul 27]. Available from: https://www.nice.org.uk/about/what-we-do/patient-access-schemes-liaison-unit
- NHS England. Cancer drugs fund. 2018 [cited 2018 Jul 27]. Available from: https://www.england.nhs.uk/cancer/cdf/.
- National institute for Health and Care Excellence (NICE). Highly specialised technologies guidance. 2018 [cited 2018 Jul 27]. Available from: https://www.nice.org.uk/about/what-we-do/our-programmes/nice-guidance/nice-highly-specialised-technologies-guidance.
- NHS Employers. NHS terms and conditions (AfC) pay scales - Hourly. 2018 [cited 2018 Jul 27]. Available from: http://www.nhsemployers.org/your-workforce/pay-and-reward/agenda-for-change/pay-scales/hourly.