ABSTRACT
Background: Corticosteroids may be temporarily effective for ulcerative colitis (UC), but long-term use increases the risk of adverse drug reactions.
Objective: The goal of the study was to examine steroid use in remission induction therapy after diagnosis of UC.
Study Design: A retrospective observational study using the Japan Medical Data Center (JMDC) Claims Database from January 2008 to December 2014.
Setting: Clinics, university hospitals, and national/public hospitals.
Intervention: Initiation of steroids after diagnosis of UC.
Main outcome measures: Start time and annual rate of steroid use, and use during the first 6 months of remission induction therapy.
Results: The subjects were 399 patients were newly diagnosed with UC in the study period. The rate of steroid use after diagnosis was 58.4% in 2009, and showed a significant decreasing trend yearly after 2010 (p ≤ 0.0001). Regarding the start time, 52.2% of patients began steroids within 60 days after diagnosis of UC. At 6 months after initiation, 23.7% continued to use steroids and 73.9% of these patients used high-dose steroids.
Conclusion: In treatment of UC after diagnosis, many patients continue to use steroids for >6 months after initiation. Reduced use of steroids based on clinical practice guidelines for UC should be promoted.
Introduction
Steroids have been used for many years as the main drug for treatment of ulcerative colitis (UC). Steroids are effective in remission induction therapy for UC and yield favorable results at 1 month after initiation, with an efficacy rate of 80% and a remission rate of 50% [Citation1–Citation5]. Steroid enema therapy and oral steroid therapy are effective in inducing remission [Citation5,Citation6], but steroids are less effective in maintaining remission [Citation2,Citation7] with either of these therapies [Citation8]. UC patients using steroids, and particularly those using high-dose steroids for a long period, are also at risk for adverse drug reactions and complications [Citation9–Citation11], including increased body weight and acne as less serious effects, and more serious complications such as reduced glucose tolerance, reduced bone density, cataract, and progression of atherosclerosis [Citation12]. In a systematic literature search on the risk-benefit profile of cumulative steroid doses after long-term treatment, the European League Against Rheumatism (EULAR) Advisory Committee found that the risks outweighed the benefits in most patients treated with steroids at 10 mg/day for at least 3 months [Citation13]. Similarly, a cumulative prednisolone-equivalent dose of ≥3,000 mg in the first year after diagnosis of inflammatory bowel disease (IBD) is a strong predictor of surgical intervention in later years [Citation14].
These findings show that steroids may be temporarily effective, but subsequently may make patients steroid-dependent, resulting in long-term treatment and increased risks of adverse drug reactions. Therefore, it is important to withdraw steroids as early as possible in treatment of UC. In general, physicians assess the effects of steroids at 1 or 2 weeks after initiation of treatment, and reduce the dose by 5 mg/week until it reaches 20 mg/day, and then by 2.5 mg/week [Citation8]. However, the details of subsequent tapering of steroid withdrawal differ among patients, and the optimal dosing and tapering methods after remission are unknown, with only empirical rules stated in guidelines [Citation15,Citation16].
Japanese treatment guidelines [Citation17] recommend that patients with moderate UC receive oral prednisone (PSL) at a starting dose of 30 to 40 mg/day. After remission is achieved, the response is evaluated within 1 to 2 weeks and if effective, the dose of PSL is reduced. The tapering method has not been defined, but it is generally recommended to reduce the dose by 5 mg/week or 10 mg/2 weeks until reaching 20 mg/day and then by 5 to 10 mg/2 weeks. The goal of UC treatment is to maintain remission for as long as possible while limiting the use of steroids to the minimum required and switching from steroids to 5-aminosalicylic acid (5-ASA) preparations, thiopurines, or anti-tumor necrosis factor-alpha (anti-TNFα) agents. In recent years, steroid-sparing effects of several drugs have been shown in randomized controlled trials [Citation18,Citation19], but it is unclear if use of immunomodulatory agents or biological agents for patients with UC reduces the dose of steroids in real-world clinical settings.
A retrospective observational study on the descriptive epidemiology of UC and risk factors for UC development has been conducted in Japan by a study group of the Ministry of Health, Labour and Welfare (MHLW), but there has been no systematic report on drug treatment in clinical settings, especially on use of steroids, and thus actual situations in UC treatment have not been clarified. Therefore, it is important to evaluate use of steroids in clinical settings. We thus investigated usage of steroids for remission induction therapy in patients who had incipient UC using the receipt database of the Japan Medical Data Center (JMDC), which is the most common database used in private hospitals. The goal of the study was to evaluate differences between clinical practice and treatment guidelines, with a focus on the starting and withdrawal dates of steroids, and to examine related factors.
Methods
Study design and data source
A retrospective observational cohort study was performed in UC patients enrolled in the JMDC Database. This database consists of lists of insured persons from 50 or more health insurance societies; the members’ claims data for inpatient, outpatient, and dispensing services; and annual health checkup data. All data have unique identification (ID) numbers created for each insured person, and it is possible to follow each person even if he/she was transferred to another hospital or visited multiple medical institutions. The only exception is for patients who change their health insurance society. We used no personally identifiable information, and data were anonymized with no correspondence table. The database contains demographic information (sex, age, size of medical institution), and data have been accumulated since January 2005. The JMDC database contains data of approximately 3 million patients. This accounts for about 4% of the total population of Japan and about 10% of all subscribers to health insurance associations in Japan.
Therefore, this study is exhaustive because the JMDC database covers a large proportion of the population. The database contains medical and prescription claims data with diagnoses coded using the International Classification of Diseases 10th revision (ICD-10) classification [Citation20]. and Japan-specific standard disease codes [Citation21], drug prescription information coded using the Anatomical Therapeutic Chemical Classification System (ATC), and clinical procedures defined under Japan-specific standardized procedure codes [Citation22,Citation23].
Ethical considerations
The study protocol was submitted to the Research Ethics Committee of Kitasato Institute Hospital. The study was exempted from ethical review because the data were anonymized by the database provider and personal information was not identifiable.
Study population
Patients with a new diagnosis of UC based on ICD-10 from 1 January 2008 to 31 December 2014 who could be followed for 12 months after initiation of steroids were identified using medical and prescription claims data. Inclusion in the study also required the absence of a definite diagnosis of UC in claims data in the 12 months before the day of diagnosis of UC. A confirmed diagnosis was defined as diagnosis without ‘suspicion’.
Definitions of variables and outcomes
Diagnosis of UC was based on ICD-10 code K51 and the new diagnosis was confirmed by the absence of suspicious flags in the database. Prescription claims data were searched to identify prescription rates of drugs with indications for UC approved in Japan that were prescribed at least once each year, using ATC codes for 5-ASA preparations (A07E-), steroids (H02A-, A07E-), immunomodulatory agents (L01B-, L04X-), and anti-TNFα agents (L04B-). The prescribed dose of steroids was also recorded. Demographic data were collected for age, sex, and management structure of medical institutions (university hospitals, national/public hospitals, hospitals with ≥20 beds, and clinics with <20 beds).
Prescription of steroids
The prescription rate of steroids was evaluated for each year. The annual prescription rate was defined as the percentage of patients with a diagnosis of UC who were prescribed steroids at least once and could be followed up for 12 months. Annual prescription rates of other drugs were defined as the percentages of patients with a steroid prescription who were prescribed immunomodulatory agents or anti-TNFα agents at least once. The period (months) to the first steroid prescription was evaluated among patients with a diagnosis of UC who could be followed up for 13 months (including the month of enrollment). Six months after the start of prescription of steroids was defined as remission induction therapy and the time until withdrawal of steroids was examined. Patients who were given steroids again within the study period after withdrawal of steroids were excluded from the study. All prescribed doses of steroids were calculated as the prednisolone-equivalent (mg) [Citation24] and the rate of administration of high-dose steroids during the 6-month period was assessed. A high dose was defined as a total prescribed dose ≥1500 mg over 6 months after initiation of steroids. The cut-off value of 1500 mg was used because the total dose of steroids should be about 1500 mg over 6 months if steroids were started at 30 to 40 mg/day, which is the common dose in treatment of UC, followed by subsequent tapering of the dose with the general schedule.
Statistical analysis
The trend in the annual rate of steroid treatment for patients newly diagnosed with UC was evaluated using a Cochran-Armitage test. Unidirectional changes over time in annual rates of steroid treatment were also examined. The time to withdrawal of steroids in patients newly diagnosed with UC during the 6 months after initiation of steroids was determined using the Kaplan-Meier method, with a monthly dose of steroids of 0 mg indicating that an event had occurred. If the prescribed dose of steroids was not reduced to 0 mg at 6 months, the patient was censored on that day. The time to withdrawal of steroids was assessed using a generalized Wilcoxon test. Logistic regression analysis was used to assess factors that may affect high-dose prescription of steroids (defined as ≥1500 mg of the total prescribed dose of steroids over 6 months after initiation of steroids), including management structure of medical institution, sex, concomitant use of immunomodulatory agents, and age at diagnosis of UC. Explanatory variables were gradually established. Patients treated in two or more institutions were included in the larger institution. The two-sided level of significance was 0.05. Statistical analysis was performed with SAS v.9.2 (SAS Inc., Cary, NC, USA).
Results
Study population
There were a total of 2,873 patients in the database who visited medical institutions and were newly diagnosed with UC from January 2008 to December 2014. These patients had a mean age of 38.1 ± 12.3 years, and included 1,759 men (61.2%) and 1,114 women (38.8%). A total of 1,274 patients (44.3%) were diagnosed in clinics with <20 beds, followed by 1,115 diagnoses (38.8%) in hospitals with ≥20 beds, 279 (9.7%) in national/public hospitals, and 205 (7.1%) in university hospitals (). There were 399 patients who could be followed up for 12 months after initiation of steroids. These patients had a mean age of 36.2 ± 13.1 years, and included 240 men (60.2%) and 159 women (39.8%) ().
Table 1. Characteristics of patients newly diagnosed with UC.
Table 2. Demographics and characteristics of patients with UC who could be followed for 1 year after initiation of steroid use.
Prescription of steroids
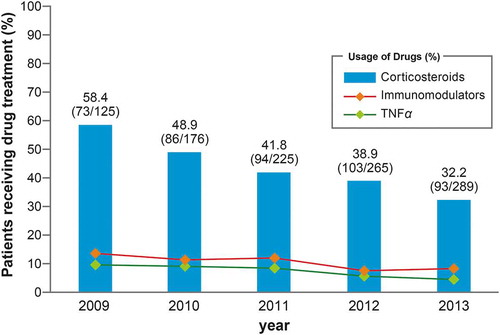
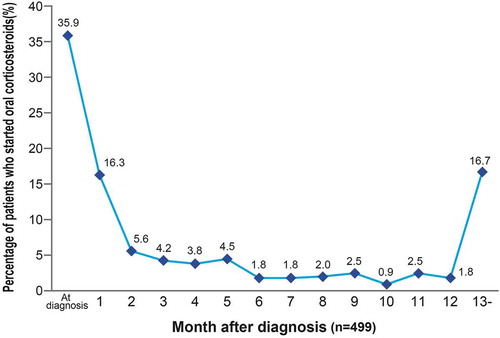
During the study period, the trend in annual prescription rates of steroids showed a significant decrease year-by-year (p < 0.001, Cochran-Armitage test) (). There was no sex difference in these prescription rates, and both sexes showed a significant yearly decreasing trend. The respective yearly rates of prescription of steroids from 2009 to 2013 were 50.7% (35/69), 48.3% (55/114), 40.3% (52/129), 39.3% (70/178), and 33.5% (60/179) for males; and 67.9% (38/56), 50.0% (31/62), 43.8% (42/96), 37.9% (33/87), and 30.0% (33/110) for females. There were no trends for increases in prescription rates of immunomodulatory agents and anti-TNFα agents for patients with steroid prescriptions (). In the period before steroid prescription reached its peak within 30 days after diagnosis of UC, the prescription rate was 35.9%. Following diagnosis of UC, 52.2% of patients started steroids within 60 days. The rate of initiation of steroid treatment in the next 6 months decreased, and the prescription rate was 16.7% after ≥13 months ().
Tapering of steroids
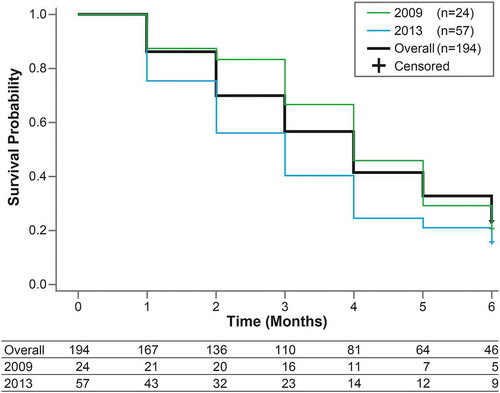
Steroids were withdrawn in 194 patients within 6 months after the prescription start of steroids (). In the 6 months after initiation of steroids, the mean time to withdrawal of steroids was 3.9 ± 0.1 months. Thus, about 4 months were required for half of the patients to withdraw from steroids. At 6 months, 46 patients (23.7%) with a mean age of 36.1 ± 14.1 years continued to use steroids (). In these patients, the median cumulative dose over 6 months was 2,058 mg, and 34 (73.9%) received a high dose of steroids of ≥1500 mg/6 months (). The time to withdrawal of steroids was significantly shorter in 2013 compared with that in 2009 (p = 0.0427, Wilcoxon test) ().
Table 3. Demographics and characteristics of patients who continued to use steroids for 6 months.
High dose of steroids
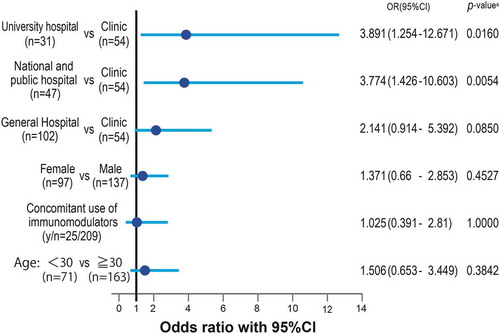
Prescription of a high dose of steroids (≥1500 mg/6 months) was influenced by the management structure of medical institutions. University hospitals and national/public hospitals prescribed significantly higher doses of steroids compared with clinics with <20 beds (3.9- and 3.8-times higher, respectively). General hospitals with ≥20 beds tended to prescribe higher doses of steroids compared with clinics with <20 beds, but the 2.1-times higher difference was not significant (p = 0.0850) ().
Discussion
To our knowledge, this is the first retrospective study in which use of steroids for patients with UC in real-world settings has been investigated using a claims database in Japan. The added value of this study is the evaluation of differences between actual steroid use after diagnosis of UC and recommendations in treatment guidelines [Citation17], which may be useful for promoting the appropriate use of steroids.
The annual rate of patients with a new diagnosis of UC and at least one steroid prescription showed a significant decreasing trend year-by-year, and prescription of immunomodulatory drugs or anti-TNFα agents in patients receiving steroids did not increase. Treatment guidelines for UC were published in 2006 and are commonly used to support clinical decision making by physicians in gastrointestinal medicine who often examine inflammatory bowel disease. Widespread use of treatment guidelines according to evidence-based medicine has had a major effect on decreased prescription of steroids for patients with incipient UC.
The general goal of UC treatment is to control inflammation without causing refractory UC (steroid-dependent or steroid-resistant UC). Therefore, drugs that facilitate long-term control of symptoms after remission are selected for treatment of UC. These drugs include salicylazosulphapyridine (SASP), 5-ASA preparations, and local treatment [Citation25]. Clinical practice guidelines for UC recommend that oral 5-ASA preparations or local treatment should be first-line therapy for mild to moderate UC, and that steroids should not be first-line therapy, despite their efficacy for inducing remission [Citation8]. Use of these guidelines in clinical settings may have decreased steroid prescription for patients with early-stage UC. During the study period, about 50% of patients started steroids within about 60 days after diagnosis of UC. In a database study, Targownik et al. found that steroid prescription among patients with IBD reached a peak of 16.8% within 60 days after diagnosis [Citation14]. Despite the rate of prescription at the peak time differing from that in our study, the two studies show similar trends.
Steroids are generally used for UC of moderate or greater severity, but patients with mild UC who have a severe inflammatory response may also receive prednisolone [Citation17]. The first therapy after diagnosis of UC achieves remission rates of 38% and 92% for severe and mild UC, respectively, showing a clear relationship with disease severity [Citation26,Citation27]. There are also many patients with UC who do not need intensive treatment in the remission phase after successful remission induction therapy [Citation28,Citation29]. Therefore, remission induction therapy is valued in treatment of UC, and this concept of top down therapy, in which intensive therapy including steroids is started shortly after diagnosis of UC [Citation25], may result in steroids being used early after diagnosis.
The period of remission induction therapy varies by drug. For steroids, this period should be 3 months, based on the standard starting dose and subsequent tapering therapy. In this study, 23.7% of patients continued to use steroids after 6 months, indicating use of steroids for maintaining remission. Among these patients, the median cumulative dose of steroids was 2,058 mg, showing that a high dose of steroids was prescribed during the 6-month period. The Japanese treatment guidelines for UC recommend that patients should switch to treatment for severe or steroid-resistant UC if a certain response cannot be achieved within 1 to 2 weeks after oral prednisolone is started. These guidelines also recommend that the efficacy of steroids should be assessed early, and even if effective, patients should switch to other drugs to avoid long-term use of steroids, given the risk of steroid-resistant UC or onset of adverse reactions [Citation17].
The European Crohn’s and Colitis Organization recommends that patients with steroid-resistant UC who have disease activity after receiving prednisolone at 0.75 mg/kg/day for 4 weeks and patients with steroid-dependent UC whose dose cannot be reduced to a prednisolone-equivalent dose of ≤10 mg/day without disease flare within 3 months after initiation of steroids should consider additional treatment or switch to another treatment [Citation30]. Based on a literature search, the EULAR Advisory Committee stated that the risks outweigh the benefits for most patients treated with a steroids at 10 mg/day for at least 3 months [Citation13] and recommended that patients should stop steroids within 6 months after initiation [Citation31]. It has also been reported that steroids are ineffective in maintaining remission [Citation2,Citation7] and that low-dose steroids and placebo did not differ in maintenance of remission and were ineffective in preventing disease flare [Citation2]. Since long-term use of steroids may cause various adverse reactions [Citation9–Citation12], limitation of steroids and switching to non-steroidal therapy are important.
After examining factors that affect prescription of high-dose steroids (total prescribed dose of ≥1500 mg over 6 months after initiation of steroids), we found that the management structure of medical institutions was the only important factor. Medical institutions with a large management structure had a greater risk of prescribing a higher dose of steroids. Changing hospital had less effect on the results because 91.2% of patients were treated in the same hospital for 6 months. Since UC is designated as an intractable disease in Japan, patients with UC often visit large hospitals where there are many specialists. Since high steroid use is related to high disease activity [Citation32], the relationship of a large management structure with the prescription rate may be due to patients with relatively aggressive disease progression more often receiving treatment in these hospitals. However, if remission induction therapy is successful, intensive therapy is often not needed in the subsequent remission phase [Citation28,Citation29]. Treatment and management of patients with early-stage UC is a key factor for avoiding refractory UC and uncontrollability [Citation25], and larger medical institutions may prescribe high-dose steroids for inducing rapid remission. A high dose of steroids has also been reported to be more common in male patients [Citation14], but sex was not associated with prescription of high-dose steroids in the current study. Use of high-dose steroids during the first year after diagnosis of IBD is a strong predictor of surgical intervention in later years [Citation14], which indicates that close attention should be paid to the steroid dose from the start of treatment.
There are several limitations in this study. First, since a claims database was used for data collection, we could not obtain clinical records for items such as the disease that caused prescription of steroids and the severity assessment for UC. Therefore, we could not follow the disease course, severity of UC, response to steroids, and clinical manifestations. Second, patients who had national health insurance were not included due to the characteristics of the database. There were also few patients aged ≥65 years old because only a few such patients are included in health insurance societies. However, the peak age of UC incidence is <30 years in Japan [Citation33] and few patients aged ≥65 years have new-onset UC; therefore, the nature of the claims database was not considered to have affected the results of the study. Third, since the baseline demographics were limited, there is a need for a study in different age groups and taking into consideration comorbidities and factors such as biologics.
Patients with severe conditions who are forced to retire from work may withdraw from health insurance societies, but large-scale receipt data are not greatly biased by events associated with specific medical institutions or specialists. In this study, data were evaluated for 399 patients with initial UC who were treated by general practitioners and university hospitals. Data collection is difficult because UC is a relatively uncommon disease and the number of patients is small. However, we obtained sufficient data for analysis from the receipt database, and the results of the study show the actual status of prescription in clinical practice and can be generalized.
Conclusion
In real-world treatment after diagnosis of UC, the prescription rates of steroids showed a decreasing trend year-by-year in Japan. However, we found that some patients continued to use steroids for more than 6 months, indicating that these drugs were used for remission maintenance therapy. A further study is required to investigate whether early use of steroids after diagnosis of UC affects the future dose of steroids, the onset of adverse reactions, and the risk for surgical intervention related to UC.
Disclosure Statement (COI Disclosure)
MO: There is no conflict of interest to be disclosed.
HO: Has received grant/research support or consulting fees from Boston Scientific Japan K.K., Otsuka Pharmaceutical Co., Ltd., Kyorin Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Ltd., Pfizer Japan Inc., Astellas Pharma Inc., Mitsubishi Tanabe Pharma Corporation, Takeda Pharmaceutical Co., Ltd., Mochida Pharmaceutical Co., Ltd., Zeria Pharmaceutical Co., Ltd., and EA Pharma Co., Ltd.
YY: There is no conflict of interest to be disclosed.
Acknowledgments
We thank the JMDC for providing the claims database and for useful advice.
References
- Faubion WA Jr, Loftus EV Jr, Harmsen WS, et al. The natural history of corticosteroid therapy for inflammatory bowel disease: a population-based study. Gastroenterology. 2001;121:255–9.
- Truelove SC, Witts LJ. Cortisone in ulcerative colitis: final report on a therapeutic trial. Br Med J. 1955;2:1041–1048.
- Summers RW, Switz DM, Sessions JT Jr, et al. National cooperative Crohn’s disease study: results of drug treatment. Gastroenterology. 1979;77:847–869.
- Powell-Tuck J, Bown RL, Lennard-Jones JE. A comparison of oral prednisolone given as single or multiple daily doses for active proctocolitis. Scand J Gastroenterol. 1978;13:833–837.
- Ford AC, Bemstein CN, Kham KL, et al. Glucocorticosteroid therapy in inflammatory bowel disease: systematic review and meta-analysis. Am J Gastroenterol. 2011;106:590–599.
- Marshall JK, Irvine EJ. Rectal corticosteroids versus alternative treatments in ulcerative colitis: a meta-analysis. Gut. 1997;40:775–781.
- Lennard-Jones JE, Misiewicz JJ, Connel AM, et al. Prednisone as maintenance treatment for ulcerative colitis in remission. Lancet. 1965;1:188–189.
- Matsuoka K, Kobayashi T, Ueno F, et al. Evidence-based Clinical Practice Guideline for Inflammatory Bowel Disease. J Gastroenterol. 2018;53:305-353.
- Benchimol EI, Seow CH, Steinhart AH, et al. Traditional corticosteroids for induction of remission in Crohn’s disease. Cochrane Database Syst Rev. 2008;16(2):CD006792.
- Curtis JR, Westfall AO, Allison J, et al. Population-based assessment of adverse events associated with long-term glucocorticoid use. Arthritis Rheum. 2006;55:420–426.
- Hoes JN, Jacobs JW, Verstappen SM, et al. Adverse events of low- to medium-dose oral glucocorticoids in inflammatory diseases: a meta-analysis. Ann Rheum Dis. 2009;68:1833–1838.
- Huscher D, Thiele K, Gromnica-Ihle E, et al. Dose-related patterns of glucocorticoid-induced side effect. Ann Rheum Dis. 2009;68:1119–1124.
- Strehl C, Bijlsma JWJ, de Wit M, et al. Defining conditions where long-term glucocorticoid treatment has an acceptably low level of harm to facilitate implementation of existing recommendations: viewpoints from an EULAR task force. Ann Rheum Dis. 2016;75:952–977.
- Targownik LE, Nugent Z, Singh H, et al. Prevalence of and outcomes associated with corticosteroid prescription in inflammatory bowel disease. Inflammatory Bowel Dis. 2014;20:622–630.
- Lichtenstein GR, Abreu MT, Cohen R, et al. American gastroenterological association institute technical review on corticosteroids, immunomodulators, and infliximab in inflammatory bowel disease. Gastroenterology. 2006;130:940–987.
- Kornbluth A, Sachar DB. Ulcerative colitis practice guidelines in adults: American college of gastroenterology, practice parameters committee. Am J Gastroenterol. 2010;105:501–523.
- Research study of intractable inflammatory bowel disease by Ministry of Health, Labour and Welfare research group (Suzuki group): Diagnostic criteria and treatment guidelines for ulcerative colitis and Crohn’s disease, Supplemental volume, Annual report; 2016. (revised in July 2017).
- Bernstein CN, Blanchard JF, Rawsthorne P, et al. Epidemiology of Crohn’s disease and ulcerative colitis in central Canadian province: a population-based study. Ann J Epidemiol. 1999;149:916–924.
- Kozyrskj AL, Mustard CA. Validation of an electronic, population-based prescription database. Ann Pharmacother. 1998;32:1152–1157.
- Vestergaard P, Mosekilde L. Fracture risk in patients with celiac disease, Crohn’s disease, and ulcerative colitis: a nationwide follow-up study of 16,416 patients in Denmark. Am J Epidemiol. 2002;156:1–10.
- Komiyama T, Yajima T, Kubota R, et al. Lower doses of 6-mercaptopurine/azathioprine bring enough clinical efficacy and therapeutic concentration of erythrocyte 6-mercaptopurine metabolite in Japanese IBD patients. J Crohns Colitis. 2008;2:315–321.
- Kakuta Y, Naito T, Onodera M, et al. NUDT15 R139C causes thiopurine-induced early severe hair loss and leukopenia in Japanese patients with IBD. Pharmacogenomics J. 2016;16:280–285.
- The Medical Procedure Index. Health service bureau, ministry of health, labor and welfare. [cited 2016 Jun 10]. Available from: http://www.iryohoken.go.jp/shinryohoshu/downloadMenu/
- Brunton L, Knollman B, Hilal-Dandan R. Goodman and Gilman’s the pharmacological basis of therapeutics. 13th ed. McGraw-Hill Education/Medical Press; 2017.
- Fukushima T., editor. Crohn’s and Colitis Foundation of Japan (CCFJ): clinical practice guidelines for ulcerative colitis. 3rd ed., Bunkodo Press; 2016
- Andres PG, Friedman LS. Epidemiology and the natural history of inflammatory bowel disease. Gastroenterol Clin North Am. 1999;28:255–281.
- Sartor RB, Sandborn WJ, et al., Editor. Kirsner's textbook of inflammatory bowel disease. 6th ed., Saunders, 2004;280-288.
- Hibi T, Inoue N, Ogata H, et al. Introduction and overview: recent advances in the immunotherapy of inflammatory bowel disease. J Gastroenterol. 2003;38(Suppl):36–42.
- Ilntckyj A, Shanahan F, Anton PA, et al. Quantification of the placebo response in ulcerative colitis. Gastroenterology. 1997;112:1854–1858.
- Dignass A, Eliakim R, Magro F, et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 1: definitions and diagnosis. J Crohns Colitis. 2012;6:965–990.
- Smolen JS, Landewé R, Breedveld FC, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis. 2014;73:492–509.
- Solberg IC, Vatn MH, Hoie O, et al. Clinical course in Crohn’s disease: results of a Norwegian population-based ten-year follow-up study. Clin Gastroenterol Hepatol. 2007;5:1430–1438.
- Japan intractable disease inflammation center. [cited 2018 Aug 1]. Available from: http://www.nanbyou.or.jp/entry/62