ABSTRACT
Introduction: Numerous real-world studies have compared non-vitamin K antagonist oral anticoagulants (NOACs) with vitamin K antagonists (VKAs) in patients with non-valvular atrial fibrillation (NVAF). A meta-analysis was performed to synthesize the available evidence.
Methods: Systematic searches were performed through 12/2016 to identify non-randomized NVAF studies comparing NOACs with VKAs, and reporting effectiveness, safety, or persistence.
Results: Of 562 citations identified, 49, 79, and 18 compared rivaroxaban, dabigatran, and apixaban, respectively, with VKAs and were included. Compared with VKAs, rivaroxaban was associated with a reduced risk of ischemic stroke (IS) (hazard ratio [HR] = 0.83, 95% confidence interval [CI] = 0.75–0.93), intracranial haemorrhage (ICH) (HR = 0.69, 95% CI = 0.52–0.90), and non-persistence (HR = 0.62, 95% CI = 0.60–0.65). Dabigatran was associated with a significantly lower risk of IS (HR = 0.80, 95% CI = 0.65–0.98) and ICH (HR = 0.45, 95% CI = 0.36–0.58), but not for non-persistence (HR = 0.91, 95% CI = 0.53–1.55), compared with VKAs. Apixaban was associated with a lower risk of ICH than VKAs (HR = 0.41, 95% CI = 0.28–0.60), but was not different to VKAs in terms of IS (HR = 1.01, 95% CI = 0.87–1.17) or non-persistence (HR = 1.08, 95% CI = 0.81–1.45).
Conclusion: NOACs appear to be at least as effective and safe as VKAs for stroke prevention in patients with NVAF.
Introduction
Non-valvular atrial fibrillation (NVAF) refers to atrial fibrillation (AF) not accompanied by rheumatic mitral valve disease, prosthetic heart valve, or valve repair [Citation1]. NVAF is the most common type of AF in the developed countries, with major etiological factors including hypertension, atherosclerotic heart disease, congestive heart failure, and diabetes mellitus [Citation2]. Although AF is often asymptomatic, patients may present with symptoms that impair their quality of life, such as discomfort, palpitations, breathlessness, syncope, dizziness, reduced exercise tolerance, and chronic fatigue [Citation3]. AF can have serious cardiovascular consequences – it is associated with an approximately two- to sevenfold increase in the risk of stroke and a twofold increase in the risk of death [Citation1]. The risk of stroke increases with age, and as many as 1 in 6 ischemic strokes (ISs) occur in patients with AF [Citation1]. The prevalence of AF is 0.4–1% globally, and up to 10% in those aged over 80 years [Citation3]; it is expected to rise in the coming years [Citation2].
Anticoagulation in patients with AF aims to prevent IS. Vitamin K antagonists (VKAs) were the first anticoagulants used in patients with AF [Citation4], and for a long time remained the mainstay of therapy. Treatment with VKAs reduces the risk of stroke by two-thirds and mortality by one-quarter [Citation4]. However, VKAs require regular coagulation monitoring, with dosage adjustments as required [Citation4], and are associated with numerous drug and food interactions [Citation5]. Non-vitamin K antagonist oral anticoagulants (NOACs) do not require regular coagulation monitoring [Citation4], and their clinical benefit in patients with NVAF is well established, following the results of randomized controlled trials (RCTs) (ROCKET AF [Citation6], RE-LY [Citation7] ARISTOTLE [Citation8], and ENGAGE AF-TIMI 48 [Citation9]), in which they demonstrated similar or better efficacy compared with VKAs [Citation6–Citation9], accompanied by a reduction in haemorrhagic strokes (HSs) [Citation7–Citation9] and intracranial haemorrhage (ICH) [Citation6–Citation9]. Consequently, recent European Society of Cardiology (ESC) guidelines have recommended NOACs to be initiated in preference to VKAs in eligible patients with NVAF [Citation4].
In addition to a substantial body of evidence from RCTs, the emerging real-world evidence (RWE) on NOACs represents an opportunity to demonstrate their impact on everyday clinical practice. VKAs are known to be drugs that perform well in RCTs. However, due to the requirement for regular coagulation monitoring and the potential for drug–food interactions, VKAs are thought to be less efficient in real-world settings. Moreover RWE provides information on outcomes that may not be considered in RCTs, such as persistence. This paper aims to synthesize the large quantity of RWE available to evaluate the performance of the NOACs (rivaroxaban, dabigatran, and apixaban) compared with VKAs in patients with NVAF, by conducting a meta-analysis of the available evidence.
Methods
A systematic review of RWE studies enrolling patients with NVAF was the basis for this meta-analysis. The methodology of the review adhered to the guidance from the Centre for Reviews and Dissemination (CRD) from the University of York [Citation10] and the Cochrane Handbook for Systematic Reviews of Interventions [Citation11]. Detailed results of the SLR were published separately [Citation12].
The population of interest was adults (aged ≥18 years) with NVAF receiving an oral anticoagulant. Both studies reporting on incident (i.e., beginning anticoagulant treatment) and prevalent (i.e., continuing treatment) patients were included. The interventions of interest were the Factor Xa inhibitors apixaban and rivaroxaban and the direct thrombin inhibitor dabigatran.
The following databases were searched on 1 December 2016: Medline and Embase (accessed using the Ovid platform), and the Cochrane Library (accessed via Wiley Interscience), including the Cochrane Database of Systematic Reviews (CDSR), Database of Abstracts of Reviews of Effects (DARE), the Cochrane Central Register of Controlled Trials (CENTRAL), the Health Technology Assessment (HTA) database, and the NHS Economic Evaluation Database (NHS EED). No restrictions were applied in terms of publication date, language, or geographical scope. Details of the search strategy are presented in the Supplementary Material.
Two independent reviewers performed the study selection, and any differences were resolved by a third reviewer. Extracted data were those on citation characteristics, study details, patient characteristics, results, and study limitations; all extracted data were quality-checked by a second reviewer.
The outcomes of interest relating to drug efficacy were: IS, all-cause mortality, myocardial infarction (MI), venous thromboembolism (VTE), a composite of ischemic stroke or systemic embolism (IS/SE), and a composite of IS/SE/all-cause mortality. Outcomes of interest relating to drug safety were: HS, ICH, major bleeding, gastrointestinal (GI) bleeding, and any bleeding. A final outcome of interest was persistence/non-persistence, defined as a break in treatment of at least 60 days.
The following three comparisons were made: 1) rivaroxaban vs. VKAs, 2) dabigatran vs. VKAs, and 3) apixaban vs. VKAs using the inverse variance-weighted method to pool hazard ratios (HRs) and their 95% confidence intervals (CIs) in a meta-analysis. The inverse variance-weighted method was used based on a common assumption that the ln(HR) followed a normal distribution. The analysis was conducted on HRs to take into account adjustments on baseline characteristics made in each study. In line with previously published methodology [Citation13], when no HR was available the incidence rate ratio was used instead. If there were no events in one arm of a study, a continuity correction was applied, while studies with no events in either treatment arm were excluded from the analyses [Citation11]. Details of input calculations are provided in the Supplementary Material.
If results at different follow-up times were available in a study, the longest follow-up was used. Additionally, if more than one study used the same database, only the study with the highest level of precision was used (i.e., only the study for which the standard error of the log of HR was the smallest was included). For example, 21 studies assessing dabigatran vs. VKAs based on the MarketScan® database were identified. Inclusion of studies using the same database and investigating similar outcomes could lead to the same patients being repeatedly included in the analysis, which could bias the results. Analyses did not account for different doses (i.e., data for 15 mg and 20 mg rivaroxaban doses, and 110 mg and 150 mg dabigatran doses were pooled together).
Heterogeneity between studies was assessed using the p-value of the Cochrane Q test and the I-squared, as recommended by the Cochrane Handbook for Systematic Reviews of Interventions [Citation11]. Both the fixed- and random-effects models were fitted. Given the heterogeneity between study designs, results of the random-effects model are presented. Analyses were conducted using SAS 9.3®. Meta-analysis results are presented in the text as the number of studies included in the comparison (n), and HR with its [95% CI], unless otherwise indicated.
Results
From 562 citations identified through the literature search, 95 were included in the meta-analysis following detailed assessment [Citation12]. Of these, 49 compared rivaroxaban with VKA, 79 dabigatran with VKA, and 18 apixaban with VKAs (some studies concerned more than one NOAC). The number of studies presenting results for each outcome of interest varied, and is indicated for each comparison in the forest plots (–).
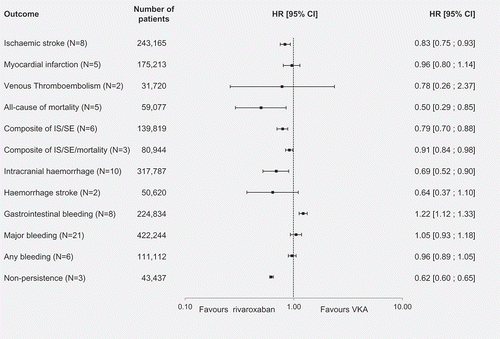
Results of the meta-analysis comparing rivaroxaban with VKAs are presented in . Based on the pooled outcomes of eight studies, rivaroxaban was associated with significantly lower risk of IS (n = 8, HR [95% CI]: 0.83 [0.75–0.93]). However, the use of rivaroxaban yielded no significant difference between the treatments for the risk of MI (n = 5) and VTE (n = 2). In the pooled results of five studies, rivaroxaban was associated with significantly lower all-cause mortality risk than VKAs (HR [95% CI]: 0.50 [0.29–0.85]). A significantly lower risk with rivaroxaban compared with VKAs was also observed for two composite endpoints: IS/SE (n = 6, HR [95% CI]: 0.79 [0.70–0.88]) and IS/SE/mortality (n = 3, HR [95% CI]: 0.91 [0.84–0.98]). Further, the use of rivaroxaban resulted in a lower risk of ICH (n = 10, HR [95% CI]: 0.69 [0.52, 0.90]). For HS (n = 2) there was no significant difference found between rivaroxaban and VKAs. In terms of notable differences in safety, VKAs were associated with a lower risk of GI bleeding than rivaroxaban (n = 8, HR [95% CI]: 1.22 [1.12–1.33]). No significant differences between the two treatments were observed in the risk of major bleeding (n = 21) and any bleeding (n = 6). Finally, lack of persistence with treatment was less of a risk with rivaroxaban than VKAs (n = 3, HR [95% CI]: 0.62 [0.60–0.65]).
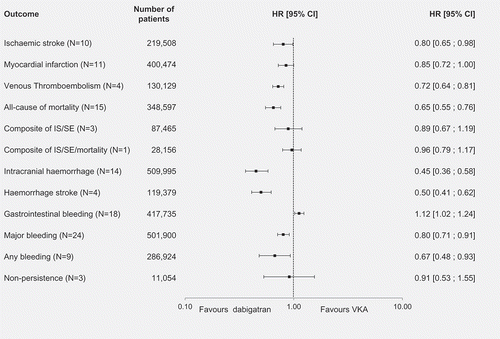
When an analogous comparison was performed between dabigatran and VKAs (), the NOAC was associated with a lower risk of IS (n = 10, HR [95% CI]: 0.80 [0.65–0.98]). No significant difference was found between the two treatments in the risk of MI (n = 11). The NOAC was also associated with a lower risk for VTE (n = 4, HR [95% CI]: 0.72 [0.64–0.81]) and all-cause mortality (n = 15, HR [95% CI]: 0.65 [0.55–0.76]). No significant difference was detected between the two treatments for two composite endpoints, IS/SE (n = 3) and IS/SE/mortality (n = 1). Dabigatran was associated with a lower risk for ICH (n = 14, HR [95% CI]: 0.45 [0.36–0.58]) and HS (n = 4, HR [95% CI]: 0.50 [0.41–0.62]). Pooled results of 18 studies suggested GI bleeding was significantly less of a risk in patients treated with VKAs than in those receiving dabigatran (HR [95% CI]: 1.12 [1.02–1.24]). Dabigatran was also associated with a lower risk in major bleeding (n = 24, HR [95% CI]: 0.80 [0.71–0.91]) and any bleeding (n = 9, HR [95% CI]: 0.67 [0.48–0.93]). No significant difference between the two therapies detected in terms of the risk of non-persistence (n = 3).
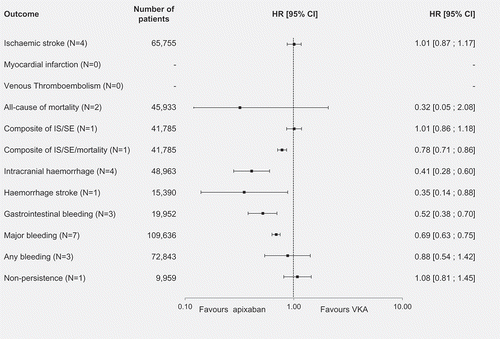
Finally, apixaban was compared with VKAs (). Meta-analyses showed that were no significant differences detected between apixaban and VKAs for IS (n = 4), all-cause mortality (n = 2) and for the composite endpoint of IS/SE (n = 1). Apixaban was associated with a significantly lower risk for the composite endpoint of IS/SE/mortality (HR [95% CI]: 0.78 [0.71–0.86]), ICH (n = 4, HR [95% CI]: 0.41 [0.28–0.60]), HS (HR [95% CI]: 0.35 [0.14–0.88]), major bleeding (n = 7, HR [95% CI]: 0.69 [0.63–0.75]) and GI bleeding (n = 3, HR [95% CI]: 0.52 [0.38–0.70]). However, the outcomes of IS/SE, IS/SE/mortality and HS, were only reported in a single study each, therefore, a meta-analysis was not conducted. Regarding other outcomes of interest, no significant differences between apixaban and VKAs were detected in terms of the risk of any bleeding (n = 3), and non-persistence (n = 1). No studies comparing MI or VTE risk between apixaban and VKAs were identified. The fact that less evidence is available for apixaban (only 18 studies comparing apixaban with VKAs were included in the present meta-analysis) probably explains why there are not as many significant results for apixaban in comparison to rivaroxaban and dabigatran.
Discussion
The SLR identified a large number of studies, highlighting a substantial interest in RWE on NOACs in NVAF. Given the large volume of evidence, a meta-analysis of the data allows us to obtain a clearer picture on the performance of NOACs in comparison with the previous, long-standing standard of care – VKAs – in the routine setting of clinical practice.
The results of the meta-analyses presented in this study were broadly similar to those of the pivotal RCTs of rivaroxaban [Citation6], dabigatran [Citation7], and apixaban [Citation8]. This meta-analysis shows that patients treated with rivaroxaban appeared at lower risk of IS or death from any cause than those treated with VKAs, while the ROCKET AF study detected no significant difference [Citation6]. On the other hand, ROCKET AF reported a significantly lower rate of HS in patients treated with rivaroxaban. It is, however, worth noting that the meta-analysis results on HS are based on only two studies. The RE-LY study tested two doses of dabigatran (110 and 150 mg) against warfarin [Citation7], while our meta-analysis pooled results for both dabigatran doses, somewhat complicating comparison of the results. In the ARISTOTLE trial, apixaban was superior to warfarin in terms of the primary endpoint of the study – prevention of stroke or SE [Citation8]. However, in a meta-analysis of two RWE studies, no significant difference was detected. Similarly, all-cause mortality, which was significantly lower in the apixaban arm than in the VKAs arm [Citation8], did not differ significantly between treatments in a meta-analysis of two real-world studies.
It is widely recognized that real-world studies provide information on the effectiveness of intervention in much more diverse populations than those included in RCTs [Citation14]. Furthermore, trial results are usually reported based on intention-to-treat analysis, whereas RWE results are usually based on on-treatment analysis. With intensive monitoring, good results were obtained with VKAs in clinical trials [Citation6–Citation8] and one may expect the benefits of NOACs – which do not require routine monitoring – to be more evident in the real-world setting, where patients are usually not monitored as closely as they are in clinical trials [Citation15]. The major strength of the present analysis was the inclusion of numerous studies, although a few outcomes analysed were only based on one single study. Furthermore, a large number of outcomes were analysed, providing, overall, a substantially broader range of information than a recently published meta-analysis on a similar topic [Citation16]. However, several methodological factors that could influence the results of this meta-analysis are worth considering.
The populations of the studies included were somewhat heterogeneous, which is important considering that the pivotal studies identified several patient and disease characteristics that affected the safety and efficacy of the NOACs analysed. In the pivotal study of rivaroxaban, no interactions were observed between these characteristics and the overall efficacy of the drug in the intention-to-treat population. However, several interactions were reported on the safety side in ROCKET AF [Citation6] and ARISTOTLE [Citation8]. To account for potential confounding of baseline characteristics, the majority of studies performed adjustments. Most of the studies reported adjusted Cox HRs, with propensity score matching, incidence rate ratio, and crude Kaplan–Meier HR also being used. Although few of the citations analysed reported unadjusted HRs, a recent publication suggested that adjustment methods may substantially influence study results [Citation17]. Work on a complementary analysis investigating the effect that different adjustment methods may have on the robustness of meta-analysis results is currently ongoing.
Considering the interventions (and comparators) used in the included studies, many studies did not report the NOAC dose used, which is a substantial limitation because – with the exception of dabigatran – different doses are indicated for different patient populations. In addition, some studies mixed incident and prevalent patients. This could pose an issue when evaluating bleeding, which usually occurs in the initial phases of anticoagulant treatment [Citation18], and so could be less common in prevalent than incident patients.
Outcome definitions varied dramatically across studies, especially for major bleeding, which sometimes pooled ICH and GI bleeding, despite NOACs showing evidence for a reduced risk of ICH and increased risk of GI bleeding [Citation19]. Persistence was also defined differently and only studies defining non-persistence as a refill gap of at least 60 days were included. Information on mortality, any bleeding and HS was not always collected, making assessment of this outcome challenging. Therefore, for the purpose of future analyses, ICH may be a more relevant outcome than HS. Information on the severity (major vs. minor) of GI bleeding was not provided. Additionally, some studies presented results at different follow-up times, in which case the longest available follow-up was used in the analysis.
Despite the methodological issues we have mentioned, an inclusive approach was used to capture the available RWE on NOACs. Consequently, studies were not selected based on the methods used to adjust HRs comparing NOACs with VKAs, International Classification of Diseases codes considered to derive outcomes of interest, or enrolment of patients following cardioversion or ablation procedures that may increase the risk of bleeding. Furthermore, both abstracts and full-text citations were analyzed. Our selection procedure aimed to avoid double-counting in studies using the same database – a major methodological issue associated with meta-analyses of RWE [Citation16]. A detailed investigation of the effects that various methodological differences between the studies included in a meta-analysis may have on the results is currently in preparation.
Real-world studies are usually far more heterogeneous than RCTs, with substantial differences between studies in designs, populations, definitions of outcomes, and other features, making a high-quality meta-analysis challenging. It is worth noting that there is little guidance available to researchers undertaking meta-analyses of real-world studies, with well-respected sources such as the CRD Guidance for Undertaking Reviews in Healthcare [Citation10], the Cochrane Handbook for Systematic Reviews of Interventions [Citation11], among others, providing only limited methodological support. Because the importance of RWE is ever-growing, the development of guidelines for pooled analysis could help prescribers, patients, and payers alike to draw better-grounded conclusions on the real-world effectiveness of healthcare interventions.
Ongoing research of RWE continues to support the use of NOACs in routine daily practice, confirming RCT findings. Real-world studies are usually far more heterogeneous than RCTs, with substantial differences between studies in designs, populations, definitions of outcomes, and other features. Whilst this can prove challenging when performing a meta-analysis, it is helpful to analyse safety and efficacy outcomes across a broad range of patients with a long follow-up time, which cannot be captured in RCTs. The availability of RWE will increase over the next few years, increasing physicians’ knowledge of how NOACs are used in daily practice and supporting the optimization of treatment in individual patients. In addition, the development of guidelines for the pooled analysis of RWE could help prescribers, patients, and payers alike, to draw better-grounded conclusions on real-world effectiveness of healthcare interventions.
Conclusion
Through an SLR, numerous studies comparing real-world effectiveness of NOACs versus VKAs were identified. However, their methodology was rather heterogeneous, leading to some conflicting results. Synthesizing these results in a meta-analysis demonstrated that NOACs are a suitable alternative to VKAs in routine clinical practice. In light of the scarce guidance for conducting meta-analyses of real-world studies, additional sensitivity analyses may improve understanding of the effect that differences in the methodologies employed by the studies included in a meta-analysis may have on the results.
Key issues
Numerous real-world studies comparing NOACs with VKAs in patients with NVAF have been published. A meta-analysis was performed to synthesize the available evidence.
Rivaroxaban and dabigatran, but not apixaban, were associated with significant reduced risks of IS compared to VKA. Rivaroxaban was also associated with a reduced risk of non-persistence versus VKAs. In this meta-analysis, all NOACs were associated with a lower incidence of ICH compared to VKAs.
NOACs appear to be at least as effective and safe as VKAs for stroke prevention in NVAF.
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary material, and in the systematic literature review published separately [Citation12].
Financial and competing interests’ disclosure
Jean-Baptiste Briere and Kevin Bowrin were employees of Bayer AG at the time of the study. Craig Coleman, Laurent Fauchier, Pierre Levy, and Olivia Wu are paid consultants who provided critical input for the study design and interpretation of results. Aurélie Millier and Vanessa Taieb are employees of Creativ-Ceutical who received funding from Bayer AG. Mondher Toumi reports no conflicts of interest.
Supplemental Material
Download MS Word (362.2 KB)Additional information
Funding
References
- Fuster V, Rydén LE, Cannom DS. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation–executive summary. Circulation. 2006;114(7):700–7.
- Bajpai A, Savelieva I, Camm J. Epidemiology and economic burden of atrial fibrillation. US Cardiol. 2007;4(1):14–17.
- Bunch TJ, Gersh BJ. Rhythm control strategies and the role of antiarrhythmic drugs in the management of atrial fibrillation: focus on clinical outcomes. J Gen Intern Med. 2011;26(5):531–537.
- Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur J Cardiothorac Surg. 2016;50(5):e1–e88.
- Compendium EM. SPC. Warfarin 0.5 mg tablets. 2017 [cited 2017 Oct]. Available from: https://www.medicines.org.uk/emc/medicine/27651
- Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883–891.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139–1151.
- Granger CB, Alexander JH, McMurray JJV, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981–992.
- Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369(22):2093–2104.
- Reviews, U.o.Y.N.C.f. and Dissemination. Systematic reviews: CRD’s guidance for undertaking reviews in health care. York, UK: Centre for Reviews and Dissemination; 2009. Available from: https://www.york.ac.uk/media/crd/Systematic_Reviews.pdf.
- Higgins JPT and Green S, editors. Cochrane handbook for systematic reviews of interventions version 5.1.0. The Cochrane Collaboration; 2011. Available from: https://training.cochrane.org/handbook.
- Briere J-B, Bowrin K, Coleman C, et al. Real-world clinical evidence on rivaroxaban, dabigatran, and apixaban compared with vitamin K antagonists in patients with nonvalvular atrial fibrillation: a systematic literature review. Expert Rev Pharmacoecon Outcomes Res. 2018;19:1–10. DOI: 10.1080/14737167.2018.1518134.
- Gillies CL, Abrams KR, Lambert PC, et al. Pharmacological and lifestyle interventions to prevent or delay type 2 diabetes in people with impaired glucose tolerance: systematic review and meta-analysis. BMJ. 2007;334(7588):299.
- Silverman SL. From randomized controlled trials to observational studies. Am J Med. 2009;122(2):114–120.
- Yao X, Abraham NS, Sangaralingham LR, et al. Effectiveness and safety of dabigatran, rivaroxaban, and apixaban versus warfarin in nonvalvular atrial fibrillation. J Am Heart Assoc. 2016;5(6):e003725.
- Proietti M, Romanazzi I, Romiti GF, et al. Real-world use of apixaban for stroke prevention in atrial fibrillation: a systematic review and meta-analysis. Stroke. 2018;49(1):98–106.
- Seeger JD, Bykov K, Bartels DB, et al. Propensity score weighting compared to matching in a study of dabigatran and warfarin. Drug Saf. 2017;40(2):169–181.
- Bassand JP, Accetta G, Camm AJ, et al. Two-year outcomes of patients with newly diagnosed atrial fibrillation: results from GARFIELD-AF. Eur Heart J. 2016;37(38):2882–2889.
- Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383(9921):955–962.