ABSTRACT
Background: Current detection methodologies are often unable to identify the location and extent of recurrent prostate cancer (PCa) leading potentially to ‘futile’ local therapies in the presence of metastatic disease. The use of 18 F-fluciclovine PET/CT may lead to better patient management.
Objective: The aim of this study was to quantify the economic impact and cost–consequence of using 18 F-fluciclovine PET/CT in PCa recurrence.
Study design: A decision analytic model based on recurrent PCa imaging guidelines.
Setting: US hospital.
Participants: PCa patients experiencing biochemical recurrence.
Intervention: 18 F-fluciclovine PET/CT was compared to conventional imaging.
Main outcome measure: Budget impact, correct diagnoses, futile treatments, and cost-consequence (cost per correct diagnosis)
Results: For a hypothetical hospital serving 500,000 individuals, the model showed the use of 18 F-fluciclovine reduced ‘futile’ therapies by 19.2%. Re-imaging costs were reduced by 40.2% ($8.2 million); however, when assuming diagnostic and staging costs only, the total costs increased from $31.2 to $34.6 million (10.9%), driven by 18 F-fluciclovine imaging agent and procedure costs. The cost per ‘correct’ diagnosis declined $30,673 (46.8%). When including subsequent 5-year patient management, the cost per ‘correct’ diagnosis declined $410,206 (49.2%).
Conclusion: 18 F-fluciclovine PET/CT imaging may improve the clinical management of men with recurrent PCa with minimal increase in healthcare spending.
Introduction
Prostate cancer (PCa) is the most commonly diagnosed cancer in men with 191,930 new cases in the USA in 2020. It is the third leading cause of cancer death among men according to the American Cancer Society [Citation1]. Initial treatment with curative intent for patients with PCa involves radical treatment with prostatectomy and/or radiation therapy. Despite significant technical advances, about 30–40% of patients will develop biochemical relapse at some time [Citation2,Citation3]. Biochemical relapse is characterized by the absence of evidence of metastatic disease on CT or SPECT (99mTc) bone scan with a concurrent rise in prostate-specific antigen (PSA) after radical therapy. Due to the dearth of radiographic disease for monitoring treatment response, it can be difficult to treat [Citation4]. Although biochemical progression-free survival is monitored using prostate-specific antigen (PSA) testing [Citation2,Citation5] at low levels, PSA testing may not detect recurrence in up to 90% of patients with PCa [Citation3].
For patients with PCa who have persistent PSA levels or PSA recurrence, the National Comprehensive Cancer Network (NCCN) recommends a number of conventional imaging modalities including magnetic resonance imaging (MRI), computed tomography (CT), or nuclear imaging using single-photon emission computed tomography (SPECT) [Citation5]. More recently, positron-emission tomography/computed tomography (PET/CT) with 11 C-choline or 18 F-fluciclovine have been added as recommended options to aid in localizing and detecting small volume recurrent or progressive PCa, when conventional imaging is negative or equivocal [Citation5]. All imaging modalities have limitations and varying sensitivity and specificity. This can result in some level of false-negative and false-positive results and potential for inappropriate therapies that may be costly and in turn impact health outcomes. There is a need for more accurate imaging to help stage patients with recurrence of PCa and to guide more personalized treatment decisions.
In 2016, the Food and Drug Administration (FDA) approved Axumin® (18 F-fluciclovine; Blue Earth Diagnostics, Ltd., Oxford, UK), a radiopharmaceutical diagnostic agent, for PET/CT in men with suspected PCa recurrence based on elevated blood PSA levels following prior treatment. Based on published detection rates for men with suspected recurrent prostate cancer, 18 F-fluciclovine is now one of the imaging agents recommended by NCCN [Citation5]. Additionally, the use of 18 F-fluciclovine PET/CT has been reported in prospective trials to change post-diagnosis patient management and treatment plans in up to 63% of patients [Citation6,Citation7].
The objective of this analysis was to quantify the economic impact and cost–consequence of using 18 F-fluciclovine PET/CT for patients with PCa to detect and localize recurrence from the perspective of an integrated delivery system (IDS) in the USA.
Materials and methods
A decision analytic model based on current NCCN Guidelines for patients with recurrent or persistent PCa was developed to simulate the economic impact and outcomes of using conventional imaging modalities or 18 F-fluciclovine PET/CT for the staging of these patients. Conventional imaging modalities include MRI, CT and SPECT (with 99Tc MDP). The model compares a base case where only conventional imaging modalities are utilized in a scenario where 18 F-fluciclovine PET/CT is included among conventional imaging options. The base case and scenario utilization of imaging modalities was informed by clinical expert opinion (). [Citation8] Clinical experts were board-certified urologists with 10 and 37 years of clinical experience from two leading academic centers (Duke Cancer Center, Durham, North Carolina and Massachusetts General Hospital, Boston, Massachusetts). NCCN guidelines also recommend 11 C-choline for imaging patients with biochemical recurrence of PCa; however, it was not included in this analysis due to a number of factors that limit its use. Firstly, the sensitivity of choline is suboptimal and its accuracy is poor in subjects with low PSA and slow PSA kinetics and, second, its short half-life means that its use is restricted to centres with an on-site cyclotron [Citation9–Citation11].
Table 1. Imaging utilization
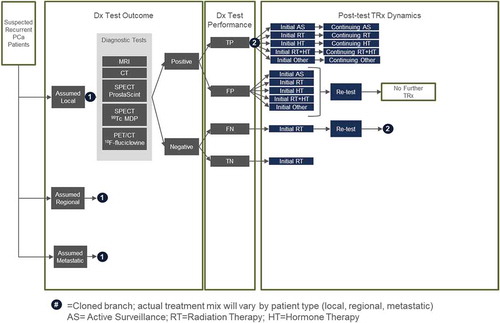
In this analysis, only 1 year of suspected incident recurrent patients enter the model with suspected local, regional (M0) or metastatic (M1) disease (). Patients will typically receive imaging tests that are interpreted as either positive or negative. Patients with positive results are subsequently prescribed a treatment plan of either active surveillance, radiation therapy, androgen deprivation therapy (ADT), combined radiation therapy and ADT, and others including prostatectomy, lymphadenectomy, cryotherapy, and brachytherapy. Patients with a true positive are assumed to receive a full course (initial = 0–12 months and continuous = 13–60 months) of the prescribed therapy. Patients with false-positive results are assumed to get a fraction of the prescribed therapy before discovering the patient was negative after retesting, leading to the cessation of therapy. Clinical experts suggested that patients with a negative imaging result are assumed to receive salvage radiation therapy to target the prostate bed ± pelvic lymph nodes based on clinical probabilities and pathologic features in order to address the potential for recurrent cancer due to the rise in PSA. Patients with a false negative are assumed to initially receive salvage radiation therapy followed by retesting [Citation12] and are subsequently prescribed an appropriate treatment plan. As a simplifying assumption in order to limit the complexity of the model, it was assumed that there were on average 3.7 cumulative tests per patient that would result in a true positive or true negative result. This assumption was deemed reasonable by clinical experts. The mix of treatments are assumed to vary by confirmed diagnosis (local, regional or metastatic). In addition to diagnosis and staging costs, the model calculates treatment costs for 1 year of incident patients. Costs for the initial treatment period (0–12 months) and continuous treatment (13–60 months) are included to provide a 5-year perspective, assuming all patients survive.
Epidemiology
Using prevalence data from SEER [Citation12] and population estimates from the US Census Bureau [Citation13] we estimated 2.6% prevalence of PCa in the adult male population. Within the prevalent pool of patients with PCa, the incidence of recurrence within the 5 years following radical prostatectomy was reported by Caire and colleagues [Citation2] to be 31.4%, an estimate utilized as a proxy to estimate the overall incidence of recurrence. The mix of patients by local regional and metastatic disease was also informed by SEER [Citation12]. Given the lack of published data, expert clinical opinion was used to calculate the patient mix after accounting for time to positively identify patients with an initial false-negative diagnosis ().
Table 2. Demographic inputs
Diagnostic test performance specifications
The model uses the sensitivity and specificity of each imaging modality to estimate the number of true positives, true negatives, false positives and false negatives to predict the health outcomes of the testing. Published clinical trial results were used to inform the test specification inputs for imaging of prostate cancer recurrent in the prostate bed and with extraprostatic involvement () [Citation8,Citation14–Citation17]. Relevant studies were identified from Medline. The results from all relevant studies were ranked with priority given (in descending order) to intra-patient comparison studies, large meta-analyses and large cohort studies. All modalities were not compared in a single head-to-head trial but were all individually compared with 18 F-fluciclovine. Schuster (2014) was used as the base comparison and the sensitivities and specificities of each modality were adjusted relative to their performance compared with 18 F-fluciclovine as observed in the respective trial results [Citation14].
Table 3. Test specifications
Clinical inputs
Recently, the impact of 18 F-fluciclovine PET/CT on treatment plans for patients with biochemical recurrence of prostate cancer was assessed in the LOCATE (Localizing Occult prostate Cancer metastases with Advanced imaging TEchniques) trial (NCT02680041), an open-label multicenter interventional trial. Trial results and outcomes, published in detail elsewhere, demonstrated that 18 F-fluciclovine PET/CT identified recurrence in the majority of a cohort of 213 men with negative conventional imaging, which often led to a change to the pre-scan treatment plan (126/213) [Citation18]. Additionally, in many cases where radiation therapy was initially recommended, the use of 18 F-fluciclovine resulted in a recommendation to modify the proposed radiation field (20 of 126 management changes) [Citation18,Citation19]. Individual patient data were retrospectively analyzed and treatment plans for patients with local, regional and metastatic prostate cancer are listed in . The initial treatment plan is assumed to be representative of decisions exclusively based on clinical features and the subsequent modifications to the treatment plan attributed to 18 F-fluciclovine PET/CT findings or results.
Table 4. Changes in patient management with conventional and 18 F-fluciclovine imaging
The model also quantifies ‘futile’ therapies. ‘Futile’ therapies are defined as any treatments that the patient did not need, for example, 1) unneeded treatment resulting from false positive test results; 2) therapies where the radiotherapy field was inappropriately designed following conventional imaging; and 3) therapies that were omitted or inappropriately planned based on false-negative imaging results. Treatment changes and changes in the radiation field were both informed by the LOCATE trial [Citation18] ().
Economic inputs
A combination of real-world data and published literature were used to provide the treatment-associated costs in the model (). An analysis of Medicare claims for men with recurrent prostate cancer was conducted using the Limited Data Set (LDS). The LDS is a random 5% sample of all Medicare claims [Citation20] that have fee-for-service coverage. We selected patient claims based on the following criteria: at least 65 years old AND Treated for PCa with surgery or radiation AND Developed a recurrence of PCa, at least 3 months post-surgery or 6 months from last day of radiation therapy AND Can be observed (continuous enrollment) from initial treatment through recurrence, for at least 2 years following recurrence. Patients with prostate cancer were identified as patients with at least one claim in the 5% LDS files (outpatient or inpatient) who had a principal diagnosis of PCa (ICD-9: 185) any time between 1/1/2010 and 9/30/2014. Initial treatments were identified by CPT and ICD-9-CM procedure codes: Surgery included radical prostatectomy (CPT 55,810, 55,812, 55,815, 55,840, 55,842, 55,845; ICD-9 CM 60.5, 60.62), laparoscopic radical prostatectomy (CPT 55,866; ICD-9 CM 17.42) and radiation (CPT 55,876, 55,875, 76,965; HCPCS: A4648; ICD-9 CM 92.27). Since there is no diagnosis code for PCa recurrence, we defined recurrence as use of one of the following imaging modalities that meets the diagnosis and timeframe criteria specified after completing initial treatment: Imaging modalities and CPT codes included: CT (72,193, 72,194, 72,195, 74,150, 74,160, 74,170, 74,176, 74,177, 74,178); MRI (72,195, 72,196, 72,197, 74,181, 74,182, 74,183); SPECT (78,803, 78,804); PET (78,811, 78,812, 78,815, 78,816); bone imaging [Citation12] (78,300, 78,305, 78,306, 78,320).
Table 5. Economic inputs
Using claims analysis, we estimated imaging procedure costs, imaging agent costs, physician costs and post-diagnosis treatment cost by initial (0–12 months following diagnosis) of recurrence and continuing (13–60 months following diagnosis) care. Due to limited data available for long-term follow-up, continuous care costs (months 13–60) were calculated as 4x the cost of the months 13–24 post-recurrence. It was not possible to differentiate costs for local and regional prostate cancer in the claims and therefore these were assumed to be identical. Costs for local and regional recurrent prostate cancer treatment were estimated for active surveillance, radiation therapy, hormone (ADT) therapy and cryotherapy.
Due to low frequency in the claims analysis, we made assumptions for the following treatment costs. Based on expert opinion, combination hormone and radiation therapy was assumed to be the sum of radiation therapy plus 50% of hormone therapy. Prostatectomy costs were based on the weighted average Diagnosis Related Group (DRG) payment for MS-DRG 665, 666, 667, published by Centers for Medicare and Medicaid Services (CMS) [Citation21]. Lymphadenectomy costs were based on the CMS payment for the HCPCS code 38571 [Citation22]. Brachytherapy costs were based on a study by Chao and colleagues [Citation23]. Metastatic treatment costs were based on a ratio of total healthcare costs for local/regional to total metastatic healthcare costs per person as determined by the Medicare claims analysis.
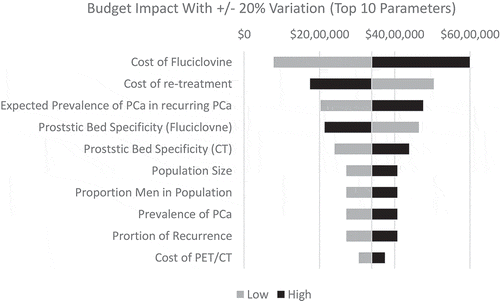
To assess the uncertainty of the model results, a deterministic sensitivity analysis (DSA) was conducted where parameters were varied by ±20% one by one while keeping the remaining variables constant (). The range was selected to keep inputs within a plausible range as seen in the literature and as advised by a clinical expert. In some cases where the 20% variation could not be applied, 100% was used for the upper and 0% was used for the lower range.
Results
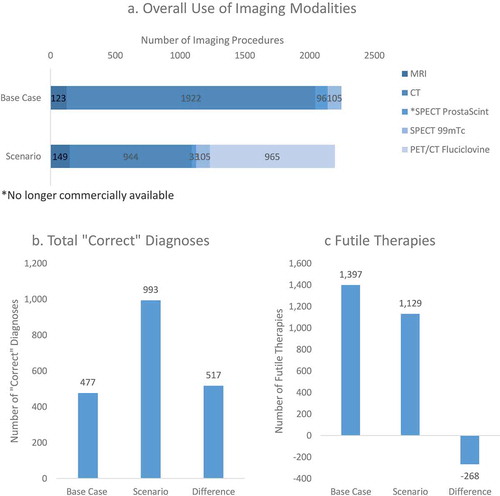
In a hypothetical US hospital system serving a population of 500,000, the model estimates 2004 men in 1 year would be expected to have recurrent prostate cancer and be subjected to diagnostic imaging. The overall use of multiple imaging modalities to guide patient management was hypothesized to decline with greater 18 F-fluciclovine use due to the test performance of 18 F-fluciclovine PET/CT. Total imaging procedures were estimated to decline by 2.2% from 2246 in the base case to 2196 in the scenario. From an outcomes perspective, the total number of ‘correct’ diagnoses (measured as the total of all true positive and true negative imaging test results) about doubled with the use of 18 F-fluciclovine, where additional 517 ‘correct’ diagnoses were accomplished. Additionally, 18 F-fluciclovine PET/CT led to a reduction of 268 (19.2%) ‘futile’ therapies from 1397 in the base case to 1129 in the scenario. illustrates the utilization and outcomes estimated by the model.
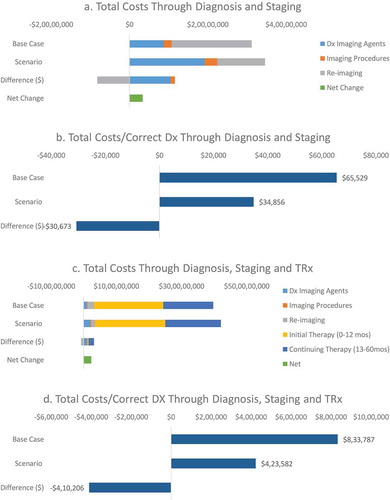
From an economics perspective, total healthcare costs through diagnosis increased by 10.9% from 31,229,484 USD in the base case to 34,622,656 USD in the scenario ()). The cost–consequence (cost per true positive and true negative imaging test) decreased by 46.8% from 65,529 USD to 34,856 USD for the base case and scenario, respectfully ()). When factoring in post-diagnosis patient management, costs also increased overall by 5.9% from 397,363,615 USD in the base case to 420,744,766 USD in the scenario ()). However, the model estimated a reduction in the cost–consequence by 49.2%% from 833,787 USD to 423,582 USD per ‘correct’ diagnoses ()). A sensitivity analysis () indicated that the top 10 parameters the model was most sensitive to included the costs of 18 F-fluciclovine, cost of retesting and assumptions for epidemiology (proportion of prostate recurrence in the population and population and demographic estimates), sensitivity and specificity of 18 F-fluciclovine.
Discussion
The purpose of this cost–consequence analysis was to assist US hospitals and formulary decision makers in evaluating the use of 18 F-fluciclovine PET/CT for diagnosis and staging of patients with recurrent prostate cancer. Furthermore, the analysis sought to examine the impact that imaging can have on costs associated with post-diagnosis treatment plans. In the scenario, the sensitivity and specificity of 18 F-fluciclovine PET/CT was expected to result in the reduced need of further imaging to guide patient management. The sensitivity and specificity relative to conventional imaging modalities resulted in more ‘correct’ diagnoses (total true positive and true negative test outcomes) thereby potentially leading to better outcomes and better patient experiences. This is especially important for patients whose PSA levels are low and conventional imaging is negative. 18 F-Fluciclovine also had a significant impact on patient management by changing the intended treatment plan to an alternative. The key changes in patient management were primarily avoidance of ADT in favor of focal radiation therapy or active surveillance where tumors were localized, and systemic therapy in cases where the disease has metastasized to distant or widespread locations. Given the shift to active surveillance, it is unclear if patients return to a therapy later on and if so, to which therapy they return. Nevertheless, in the short term, cost savings are realized from the use of alternative treatments that are less costly than ADT and potentially offer a better quality of life. For patients initially planning radiation therapy, the 18 F-fluciclovine PET/CT was used for treatment of the radiation field. In the analysis of the LOCATE data, the radiation field was predominantly expanded from simply radiation of the prostate to also include the abdomen when tumors were found at the local-extraprostatic level. Without expanding the radiation field, these local treatments would have been ‘futile.’
While the discussion above focuses on the clinical and diagnostic outcomes, the model also estimates the economics of using 18 F-fluciclovine PET/CT. As expected with the addition of a new imaging technology, total costs of imaging are expected to be higher. Total imaging costs increased by 7.4%, primarily driven by the cost of the imaging agent and the more expensive PET/CT imaging procedure when compared to conventional imaging modalities of MRI, CT or SPECT. However, increases in imaging agent and procedure costs were offset by the lower cost of repeat imaging and more ‘correct’ diagnoses (true positive and true negative results) based on using 18 F-fluciclovine PET/CT. The increase in ‘correct’ diagnoses led to reducing the cost per ‘correct’ diagnosis by 30,673 USD (46.8%) when compared to conventional imaging. When taking a more holistic view of including post-imaging treatment costs, the cost–consequence analysis indicates the total cost per correct diagnosis is lower by 410,206 USD (49.2%). This suggests that the adoption of a new imaging technology may be more efficient both in the short term and in the long term.
The DSA found the model to be sensitive to the sensitivity and specificity of 18 F-fluciclovine. The NCCN guidelines indicate that the range for sensitivity is 37–90% and 40–100% for specificity. The wide range is a result of variability in equipment, protocols, and image interpretation [Citation5]. Additionally, as seen in clinical trials, the site or location of the cancer and patient characteristics also contribute to the wide range of test performance reported in the literature [Citation8]. NCCN guidelines also recommend 11 C-choline for imaging patients with biochemical recurrence of PCa, but this too is subject to a wide range of reported sensitivity and specificity values (32–93% and 40–93%, respectively) [Citation5]. A prospective study comparing 11 C-choline to 18 F-fluciclovine indicated an 85% agreement in test outcome [Citation24].
Limitations
The univariate sensitivity analysis indicated that the model results were sensitive to the sensitivity and specificity of the imaging modalities. Although we used test specifications and their observed relative performances to 18 F-fluciclovine from the literature, uncertainty remains about the actual comparative effectiveness of the individual imaging modalities since they have not been directly tested. Additionally, positive findings occur in only ~11% of patients with biochemical recurrence [Citation25]. Some imaging procedures may be unable to detect recurrent prostate tumors <1 cm in size or in cases when PSA levels are <10 ng/mL – when cancer may be more effectively managed or treated with localized therapy [Citation9,Citation26–Citation30]. Costs from the claims analysis are based on specific definitions of patients, PCa recurrence, and claims. From a Medicare dataset, the analysis provides reliable costs for patients over 65 years old. This age threshold is consistent with the patient population of interest as 6 of 10 patients with PCa are 65 or older [Citation31]. Though reliable for a Medicare population, the costs should not be utilized when simulating economics in a younger cohort. Another limitation is that the claims do not distinguish between local and regional prostate cancer and the used of ratio for metastatic disease may not apply across all treatment plans. The claims analysis' inability to distinguish between local and regional PCa treatment costs may impact the longer term results. As reported in studies such as LOCATE, although changes in management theoretically result in more optimized and personalized treatment, it remains to be confirmed whether such changes also lead to prolonged survival and enhanced quality of life. Therefore, it was assumed that all patients survive 5 years, thereby incurring the full 5 years of treatment costs. As a result, cumulative treatment costs may be overestimated.
In summary, due to the high overall cost of treating prostate cancer, it is critical for healthcare decision makers to adopt a holistic view of associated imaging costs since the imaging is vital to guide optimal patient management. This analysis suggests that hospitals with access to 18 F-fluciclovine PET/CT for diagnosis and staging of recurring patients with PCa may reduce further imaging tests and generate improved clinical outcomes as evidenced by more ‘correct’ diagnoses and fewer ‘futile’ treatments while yielding a slight increase in spending. As this analysis does not incorporate quality-adjusted life years (QALYs), a future cost-effectiveness study may enhance future decision-making processes for hospital stakeholders.
Acknowledgments
The authors would like to thank Dr Christopher Cutie of Massachusetts General Hospital and Dr. Judd Moul of Duke Health and Duke University School of Medicine for their expert feedback on model framework and model assumptions. The authors would also like to thank Terri Wilson and Catriona Turnbull for editorial support.
Disclosure statement
David Gauden and Peter Gardiner are employees of Blue Earth Diagnostics and may own stock or other equity in the company. Ivar Jensen, Joanne Hathway and Philip Cyr are employees of Precision Xtract, which has received consulting fees from Blue Earth Diagnostics.
Additional information
Funding
References
- The American Cancer Society. Cancer facts & figures 2020, table 1. Estimated number of new cancer cases and deaths by sex, US, 2020. [cited 2020 Feb 12]. Available from: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2020/estimated-number-of-new-cancer-cases-and-deaths-by-sex-2020.pdf
- Caire AA, Sun L, Ode O, et al. Delayed prostate-specific antigen recurrence after radical prostatectomy: how to identify and what are their clinical outcomes? Urology. 2009;74(3):643–10.
- Nanni C, Schiavina R, Brunocilla E, et al. 18F-FACBC compared with 11C-choline PET/CT in patients with biochemical relapse after radical prostatectomy: a prospective study in 28 patients. Clin Genitourin Cancer. 2014;12(2):106–110.
- Spratt DE, McHugh DJ, Morris MJ, et al. Management of biochemically recurrent prostate cancer: ensuring the right treatment of the right patient at the right time. Am So Clin Oncol Educ Book. 2018;(38):355–362. DOI:10.1200/EDBK_200319.
- National Comprehensive Cancer Network. Prostate cancer (Version 4.2018). [ cited 2018 Oct 22]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf
- Andriole GL, Kostakoglu L, Chau A, et al. The impact of positron emission tomography with 18F-fluciclovine on the treatment of biochemical recurrence of prostate cancer: results from the LOCATE trial. J Urol. 2019;201(2):322–331.
- Scarsbrook AF, Bottomley D, Teoh EJ, et al. Impact of 18F-fluciclovine positron emission tomography on the management of patients with recurrence of prostate cancer: results from the FALCON trial. Int J Radiat Oncol Biol Phys. 2020 Feb 14. pii: S0360-3016(20)30203-0. doi: 10.1016/j.ijrobp.2020.01.050. [Epub ahead of print].
- Shen G, Deng H, Hu S, et al. Comparison of choline-PET/CT, MRI, SPECT, and bone scintigraphy in the diagnosis of bone metastases in patients with prostate cancer: a meta-analysis. Skeletal Radiol. 2014;43(11):1503–1513.
- Schiavina R, Ceci F, Borghesi M, et al. The dilemma of localizing disease relapse after radical treatment for prostate cancer: which is the value of the actual imaging techniques? Curr Radiopharm. 2013;6(2):92–95.
- Giovacchini G, Picchio M, Coradeschi E, et al. Predictive factors of [(11)C]choline PET/CT in patients with biochemical failure after radical prostatectomy. Eur J Nucl Med Mol Imaging. 2010;37(2):301–309.
- Szydło M, Jadwiński M, Chmura A, et al. Synthesis, isolation and purification of [(11)C]-choline. Contemp Oncol (Pozn). 2016;20(3):229–236.
- Noone AMHN, Krapcho M, Miller D, et al. (eds). SEER cancer statistics review, 1975–2015: cancer stat facts: prostate cancer. [cited 2018 Oct 22]. Available from: https://seer.cancer.gov/statfacts/html/prost.html
- USA Census Bureau. US population estimates by age and sex. 2018 Apr 19 [cited 2018 Oct 19]. Available from: https://www2.census.gov/programs-surveys/popest/datasets/2010-2017/national/asrh/?#
- Schuster DM, Nieh PT, Jani AB, et al. Anti-3-[(18)F]FACBC positron emission tomography-computerized tomography and (111)In-capromab pendetide single photon emission computerized tomography-computerized tomography for recurrent prostate carcinoma: results of a prospective clinical trial. J Urol. 2014;191(5):1446–1453.
- Akin-Akintayo OO, Jani AB, Odewole O, et al. Change in salvage radiotherapy management based on guidance with FACBC (Fluciclovine) PET/CT in postprostatectomy recurrent prostate cancer. Clin Nucl Med. 2017;42(1):e22–e28.
- Odewole OA, Tade FI, Nieh PT, et al. Recurrent prostate cancer detection with anti-3-[(18)F]FACBC PET/CT: comparison with CT. Eur J Nucl Med Mol Imaging. 2016;43(10):1773–1783.
- Bach-Gansmo T, Nanni C, Nieh PT, et al. Multisite experience of the safety, detection rate and diagnostic performance of fluciclovine ((18)F) positron emission tomography/computerized tomography imaging in the staging of biochemically recurrent prostate cancer. J Urol. 2017;197(3 Pt 1):676–683.
- Andriole GL, Kostakoglu L, Chau A, et al. The impact of positron emission tomography with (18)F-fluciclovine on the management of patients with biochemical recurrence of prostate cancer: results from the LOCATE trial. J Urol. 2018. DOI:10.1016/j.juro.2018.02.2599.
- Solanki AA. The impact of 18F-fluciclovine positron emission tomography on salvage radiation therapy decisions for patients with post-radical prostatectomy recurrence of prostate cancer: results from LOCATE. Int J Radiat Oncol Biol Phys. 2018;102(3):S161.
- Research Data Assistance Center (ResDac). [ cited 2018 Aug 21]. Available from: https://www.resdac.org/
- Centers for Medicare & Medicaid Services. FY 2018 Final rule and correction notice, tables 7A and 7B. [cited 2018 Oct 25]. Available from: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/FY2018-IPPS-Final-Rule-Home-Page-Items/FY2018-IPPS-Final-Rule-Tables.html
- Centers for Medicare & Medicaid Services. Addendum B.- OPPS payment by HCPCS code for CY 2018. [ cited 2018 Oct 25]. Available from: https://www.cms.gov/apps/ama/license.asp?file=/Medicare/Medicare-Fee-for-Service-Payment/Hospitaloutpatientpps/Downloads/2018-Oct-Addendum-B.zip
- Chao MW, Grimm P, Yaxley J, et al. Brachytherapy: state-of-the-art radiotherapy in prostate cancer. BJU Int. 2015;116(Suppl 3):80–88.
- Nanni C, Zanoni L, Pultrone C, et al. (18)F-FACBC (anti1-amino-3-(18)F-fluorocyclobutane-1-carboxylic acid) versus (11)C-choline PET/CT in prostate cancer relapse: results of a prospective trial. Eur J Nucl Med Mol Imaging. 2016;43(9):1601–1610.
- Choueiri TK, Dreicer R, Paciorek A, et al. A model that predicts the probability of positive imaging in prostate cancer cases with biochemical failure after initial definitive local therapy. J Urol. 2008;179(3):906–910. discussion 910.
- Hricak H, Choyke PL, Eberhardt SC, et al. Imaging prostate cancer: a multidisciplinary perspective. Radiology. 2007;243(1):28–53.
- Kirkham AP, Emberton M, Allen C. How good is MRI at detecting and characterising cancer within the prostate? Eur Urol. 2006;50(6):1163–1174. discussion 1175.
- Wolf JS Jr., Cher M, Dall’era M, et al. The use and accuracy of cross-sectional imaging and fine needle aspiration cytology for detection of pelvic lymph node metastases before radical prostatectomy. J Urol. 1995;153(3 Pt 2):993–999.
- Merdan S, Womble PR, Miller DC, et al. Toward better use of bone scans among men with early-stage prostate cancer. Urology. 2014;84(4):793–798.
- Ikonen S, Karkkainen P, Kivisaari L, et al. Magnetic resonance imaging of clinically localized prostatic cancer. J Urol. 1998;159(3):915–919.
- American Cancer Society. Key statistics for prostate cancer. 2019 [cited 2019 Aug 27]. Available from: https://www.cancer.org/cancer/prostate-cancer/about/key-statistics.html