ABSTRACT
Background: Recent cost-utility analysis (CUA) models for onasemnogene abeparvovec (Zolgensma®, formerly AVXS-101) in spinal muscular atrophy type 1 (SMA1) differ on key assumptions and results.
Objective: To compare the manufacturer’s proprietary CUA model to the model published by the Institute for Clinical and Economic Review (ICER), and to update the manufacturer’s model with long-term follow-up data and some key ICER assumptions.
Study design: We updated a recent CUA evaluating value for money in cost per incremental Quality-adjusted Life Year (QALY) of onasemnogene abeparvovec versus nusinersen (Spinraza®) or best supportive care (BSC) in symptomatic SMA1 patients, and compared it to the ICER model.
Setting/Perspective: USA/Commercial payer
Participants: Children aged <2 years with SMA1.
Interventions: Onasemnogene abeparvovec, a single-dose gene replacement therapy, versus nusinersen, an antisense oligonucleotide, versus BSC.
Main outcome measure: Incremental-cost effectiveness ratio and value-based price using traditional thresholds for general medicines in the US.
Results: Updated survival (undiscounted) predicted by the model was 37.60 years for onasemnogene abeparvovec compared to 12.10 years for nusinersen and 7.27 years for BSC. Updated quality-adjusted survival using ICER’s utility scores and discounted at 3% were 13.33, 2.85, and 1.15 discounted QALYs for onasemnogene abeparvovec, nusinersen, and BSC, respectively. Using estimated net prices, the discounted lifetime cost/patient was $3.93 M for onasemnogene abeparvovec, $4.60 M for nusinersen, and $1.96 M for BSC. The incremental cost per QALY gained for onasemnogene abeparvovec was dominant against nusinersen and $161,648 against BSC. These results broadly align with the results of the ICER model, which predicted a cost per QALY gained of $139,000 compared with nusinersen, and $243,000 compared with BSC (assuming a placeholder price of $2 M for onasemnogene abeparvovec), differences in methodology notwithstanding. Exploratory analyses in presymptomatic patients were similar.
Conclusion: This updated CUA model is similar to ICER analyses comparing onasemnogene abeparvovec with nusinersen in the symptomatic and presymptomatic SMA populations. At a list price of $2.125 M, onasemnogene abeparvovec is cost-effective compared to nusinersen for SMA1 patients treated before age 2 years. When compared to BSC, cost per QALY of onasemnogene abeparvovec is higher than commonly used thresholds for therapies in the USA ($150,000 per QALY).
Introduction
Onasemnogene abeparvovec (Zolgensma®) was approved by the US Food and Drug Administration for the treatment of pediatric patients less than 2 years of age with Spinal Muscular Atrophy (SMA) with bi-allelic mutations in the survival motor neuron 1 (SMN1) gene[Citation1]. SMA is characterized by degeneration of lower motor neurons in the spinal cord and brainstem, leading to weakness and muscle atrophy, loss of independent breathing and swallowing, and early death [Citation2]. In the USA (US), SMA occurs in approximately 1 in 10,000 newborns (about 500 new cases per year), making it the most common hereditary disease causing childhood death [Citation3]. Patients with the most severe form of SMA, type 1, will typically be diagnosed before six months of age when they fail to reach gross motor milestones such as rolling or the ability to sit without assistance [Citation4]. By their second birthday, around 90% of SMA type 1 patients will die or require permanent ventilator assistance (>16 hours/day) [Citation5]. In 2018, SMA was added to the Recommended Uniform Screening Panel for newborns in the US and several states have now adopted SMA newborn screening [Citation6].
As the first gene therapy approved for SMA, onasemnogene abeparvovec directly addresses the root cause of the disease by providing a functional copy of the SMN gene, restoring functional SMN protein in motor neurons, preventing neuronal cell death, and halting disease progression [Citation7]. In an open-label study (NCT02122952 [START]), 12 genetically confirmed infants with SMA type 1 received a one-time intravenous therapeutic dose of onasemnogene abeparvovec (2.0e14 vg/kg) [Citation8]. After two years of follow-up, 11 patients achieved sitting unassisted along with the ability to feed orally and the ability to speak, two patients were walking independently, and all patients had survived without the need for permanent ventilatory assistance [Citation9].
In May 2019, we published a cost-utility analysis (CUA) of onasemnogene abeparvovec compared to the current standard of care in the US, nusinersen (Spinraza®) in patients with symptomatic SMA type 1 [Citation10]. This CUA used health states based on gross motor milestones (sitting and walking independently) that were achieved by treated patients. To estimate survival and quality of life gains for patients who achieved these milestones, we used long-term observational data for patients with less severe forms for SMA (types 2 and 3) [Citation11], while costs were applied from a recent commercial claims database analysis, also using SMA types 2 and 3 as proxies for sitting and walking patients [Citation12]. The model also included a health state for permanent assisted ventilation, to capture the additional costs and impact on survival and quality of life associated with full-time reliance on mechanical ventilation [Citation13]. That model predicted that onasemnogene abeparvovec would deliver 15.65 discounted quality-adjusted life-years (QALYs) compared to nusinersen’s 5.29 and would be cost-effective using traditional US thresholds at potential prices of up to 5 USD M for a single dose of onasemnogene abeparvovec in SMA type 1 [Citation10].
One month earlier (April 2019), a CUA was published by the Institute for Clinical and Economic Review (ICER), an independent research organization that evaluates the clinical and economic value of prescription drugs in the US [Citation14]. In its analysis, ICER adopted similar model health states and used similar data sources to compare onasemnogene abeparvovec and nusinersen separately to best supportive care (BSC) (). At the time the report was published, the list price of onasemnogene abeparvovec was unknown, so ICER used a placeholder price of 2,000,000 USD per treatment. ICER concluded that the incremental cost per QALY gained for onasemnogene abeparvovec compared to BSC was 243,000 USD in symptomatic SMA type 1 patients. ICER noted that this cost per QALY is higher than traditional cost-effectiveness thresholds used in the US between (100,000 - 150,000 USD per QALY) but that payers may use higher thresholds and consider broader impacts when considering the value of treatments for ultra-rare diseases [Citation15]. To that end, ICER includes analyses with willingness to pay thresholds of up to 500,000 USD per QALY when evaluating rare diseases, while also considering impacts on families and other considerations not captured in CUA. While ICER did not compare onasemnogene abeparvovec and nusinersen directly in its base case, it did include scenario analyses comparing the two therapies assuming symptoms were present. In this analysis, ICER estimated that the incremental cost per QALY gained would be 139,000 USD at the 2,000,000 USD placeholder price.
Figure 1. Model health states – previous model vs ICER model
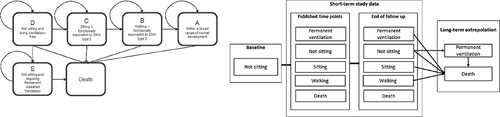
ICER also included an exploratory analysis of a gene therapy such as onasemnogene abeparvovec in presymptomatic patients, to reflect the FDA indication for all SMA (in patients <2 years of age) and in anticipation of universal newborn screening that is expected to be added in the near future. In this scenario, because there were no published clinical trial data at the time for gene therapies, ICER assumed that this “Drug X’ would be as effective as nusinersen in the presymptomatic patient population. ICER estimated that the cost per QALY gained for Drug X compared to BSC would be 157,000 USD resulting in a value-based price of up to 1.9 M USD using traditional cost-effectiveness thresholds.
In light of the ICER report, we have updated our CUA to align with updated data based on the ICER model. We also adjusted model inputs to incorporate equivalent stopping rules and hospital markups for nusinersen treatment as those used in the ICER model, as well as adding a comparison to BSC in the symptomatic and presymptomatic populations. The purpose of this report is to update the previously published CUA using assumptions adopted by ICER where appropriate, and to account for the remaining differences between both analyses.
Materials and methods
Summary of updates to the model
provides a comparison of the original model, the ICER model, and this update, and lists the inputs used in the updated model. The original model compared onasemnogene abeparvovec to nusinersen, which, at that time, was standard of care and the only disease-modifying therapy available for patients with SMA type 1 in the US. In its model, ICER compared onasemnogene abeparvovec to BSC for its base case analysis. We have added a comparison to BSC in this model update.
Table 1. Comparison between old model, new model, and ICER model
Table 2. Inputs used in model
Some input parameters in the model have been updated or amended following the ICER report to reflect the most recent available evidence base. The onasemnogene abeparvovec vs nusinersen model included published data from a randomized control trial comparing nusinersen to sham control with 13 months of follow-up (NCT02193074 [ENDEAR]). In 2018, interim efficacy and safety data for the nusinersen extension trial (NCT02594124 [SHINE]) was presented at the American Academy of Neurology conference [Citation16], with nearly 60 months of follow-up from first dose. The longer-term data for patients who received nusinersen in ENDEAR and SHINE used in the ICER model have been used in this model update. These updated data replace previous extrapolations of future projected milestones in the nusinersen arm (no milestones were extrapolated for onasemnogene abeparvovec in the previous version or the updated version of the CUA). Additionally, where our original model used natural history data (for probability of transitioning to death or permanent ventilation in the BSC arm and beyond the trial periods for non-sitting treated patients), we have adopted the source selected by ICER (the sham control arm of the ENDEAR trial) [Citation17] to replace the original source (the NeuroNext trial) [Citation18]. This sham control arm has equivalent clinical endpoints to the clinical trials of onasemnogene abeparvovec and nusinersen, although as a randomized controlled trial, it may not be as generalizable to the wider population as the previously used observational study.
In our previous publication, we noted that, while there have been a number of health-related quality of life studies conducted in children with SMA type 1, there is no clearly preferred source of utility scores because each has important limitations (including the serious challenges of measuring quality of life in small children) [Citation10]. In the earlier model base case we used EQ-5D utility scores reported from a trial of SMA type 2 patients (less severe, later onset patients who are generally able to sit independently) receiving nusinersen treatment (NCT02292537 [CHERISH]) [Citation19]. We also ran scenario analyses exploring the impacts of alternative sources. In its analysis, ICER used a variety of sources for base case utility scores for each model health state, including the NICE evaluation of nusinersen [Citation20], as well as additional utility increments in the treatment arms for achieving interim milestones. In this model update, we have adopted the utility scores and increments used by ICER.
ICER’s analysis applied a stopping rule based on a review of US commercial coverage policies that assumed that nusinersen patients who do not achieve motor milestones after 24 months or who require permanent assisted ventilation would discontinue nusinersen treatment. Our previous onasemnogene abeparvovec model did not include a stopping rule for nusinersen patients; the updated model implements the ICER stopping rule.
The updated model uses the same estimated net cost per package for nusinersen, the same administration costs and assumptions, and factors in the same hospital markups as the ICER model (previously our model used hospital markups reported by clinical experts). Now that onasemnogene abeparvovec has launched in the US, our updated model includes the reimbursement price for the gene therapy instead of placeholder prices.
The ICER model used an identical source for medical costs as our original model. While our previous model micro-costed durable medical equipment (consisting of ventilators, breathing assist devices, and types of wheelchairs), ICER added an aggregate figure to capture ‘costs associated with permanent ventilation’ for patients in the permanent assisted ventilation state, based on a published analysis in the UK (converted to USD using 2002 exchange rates and then inflated to 2017 dollars) [Citation21]. While this approach may underestimate healthcare costs in the US, to align with the ICER model we have adopted the same approach in the model update.
In our original model, we included a health state ‘A’ to represent patients ‘within a broad range of normal development’ (i.e., patients who were able to walk independently by two years of age). This state was intended to capture the curative potential of onasemnogene abeparvovec; patients in this state were assumed to have zero costs associated with SMA treatment and survival and utility scores consistent with SMA type 3 (i.e., general population) after initial treatment. In contrast, ICER did not include an A state; the ICER model ‘walking’ state had survival and utility scores consistent with SMA type 3 (i.e., those of the general population) and SMA costs also associated with SMA type 3 (lower than the other health states but not zero). In our update, we included the option in the programmable model for the user to select whether patients can enter the ‘A’ state; for the base case CUA in this report, we have not included an ‘A’ state.
The original model did not include an analysis of the presymptomatic population, as clinical trial results for onasemnogene abeparvovec in presymptomatic patients were unavailable. However, to align with the exploratory analysis conducted by ICER in ‘Drug X’, a hypothetical gene therapy used in presymptomatic patients, we have conducted similar exploratory analysis. Instead of assuming equivalent efficacy to nusinersen, we assumed efficacy of the hypothetical treatment was equivalent to onasemnogene abeparvovec patients who received treatment prior to the age of three months. Although these patients clinically were symptomatic, this subgroup of patients who received early treatment was chosen as a proxy to better represent the potential benefit of treating patients presymptomatically, as further analysis of the onasemnogene abeparvovec study group have shown that milestone gains were greatest in patients treated early (dosed before age three months) [Citation22]. For the nusinersen arm, we used published interim data from a trial of nusinersen in presymptomatic patients (NCT02386553 [NURTURE]).
Comparison to the ICER model – remaining differences
A key difference between the original model and the ICER model was that ICER assumed some onasemnogene abeparvovec patients would later receive nusinersen, whereas the original CUA did not account for sequential use. There is currently no clinical evidence of benefit of sequential therapy, although recently approved clinical trials may give insight into the possible efficacy of nusinersen after onasemnogene abeparvovec (e.g., NCT04488133). Given the lack of evidence of clinical benefit of sequential therapy and the high cost of nusinersen treatment, we have not explored sequential treatment in this analysis; once results data have been published in this cohort it would be useful to explore this. Similarly, our earlier CUA and this updated CUA does not explore outcomes of patients receiving onasemnogene after stopping nusinersen, due to lack of clinical evidence and the risk that by the age nusinersen therapy is stopped, symptomatic SMA type 1 patients may no longer be eligible for onasemnogene therapy.
In some instances, the approach taken by ICER was not consistent with the available evidence. For example, ICER assumed that one-sixth of patients who received onasemnogene abeparvovec and attained sitting would regress a milestone (i.e., lose the ability to sit independently) at the end of their short-term model (i.e., after the end of the trial period) in the comparison against BSC (they assumed no motor milestone loss in the comparison against nusinersen). This was an assumption based on three patients treated with onasemnogene abeparvovec who went on to start nusinersen after the end of the onasemnogene abeparvovec clinical trial (hence in the model comparison to nusinersen, ICER assumed no milestone loss but incorporated costs of sequential nusinersen treatment). ICER assumed that this treatment decision could have been because their health state started to deteriorate or because they did not improve as much as desired. However, this assumption is not consistent with the clinical trial data for these patients, which showed ongoing motor milestone achievements, including two additional children who were able to stand with support (four total) [Citation9]. This suggests that nusinersen was not required for these patients and provides evidence against the speculative claim that the patients would have lost motor abilities without the add-on therapy. Given that we challenge ICER’s assumption, we have assumed no loss of motor milestones in the base case CUA. However to address uncertainty in the durability of the gene therapy beyond the trial periods, we ran a scenario analysis to explore the impact on the cost-utility of onasemnogene abeparvovec in the event that effects begin to wane in the future (in this case, a speculated 25 years).
In the ICER model analysis, ‘sitting unassisted’ for the onasemnogene abeparvovec arm was defined as ‘sitting unassisted for ≥10 seconds, in accordance with WHO Motor Milestones criteria’[Citation14]. The WHO Motor Milestone criteria require that the child sits up straight without using arms or hands to balance or support their body. We consider that the WHO criteria may be too restrictive when considering very young children who, once mobile, want to reach for toys and explore. Our previous model and this update uses clinical endpoints reported by the onasemnogene abeparvovec study, which defined sitting unassisted for ≥5 seconds, as per item 22 of the Bayley Scales of Infant and Toddler Development gross motor subtest [Citation23]. We used the same thresholds for the updated CUA. For nusinersen-treated patients, the ICER report uses HINE-2 motor milestone definition of ‘sitting unassisted’, which includes ‘stable sit’ and ‘pivots’, and does not include a duration, per the reported outcomes from ENDEAR and SHINE.
Additionally, when estimating the proportion of sitting patients in the nusinersen arm in its final report, ICER assumed that, in addition to the patients observed to sit independently, patients lost to follow-up who did not attend scheduled clinic visits might have also achieved independent sitting. We consider this to be a likely overestimate of motor function improvements, so our updated model uses the same method as previously published (i.e., only patients who were observed sitting through formal evaluation transition to the sitting state).
In extrapolating survival in patients on permanent assisted ventilation, ICER used a long-term observational study of SMA type 1 patients, but excluded data for those patients who had been tracheostomized [Citation13]. In contrast, our original model used pooled data from both groups from that same study to reflect the clinical trial populations and clinical practice in the US, where tracheostomy is recommended in selected patients in whom non-invasive ventilation is insufficient or has failed [Citation24]. Tracheostomy is known to prolong survival [Citation13]. By excluding these patients, ICER may have underestimated life-years for patients in this health state. Accordingly, we did not adjust our approach, but did limit survival to a maximum of 16 years, which was the maximum observed life expectancy in that study (previously we had used 22 years, the maximum observed life expectancy in any SMA type 1 study), to prevent unrealistically protracted survival from long extrapolation curve tails from skewing the results [Citation13,Citation25].
ICER also incorporated costs associated with permanent ventilation for patients in higher functioning states (sitting or walking) immediately before death; our model does not incorporate these end of life costs since we assumed that these would have been captured in the commercial claims database analysis used for direct medical costs, and we, therefore, avoid double counting. This has a small impact on results given that these patients survive for many years and costs are discounted at 3% annually.
Some modeling differences also remain that have a small impact on the estimate of life years and QALYs. For example, for the parametric survival curves fitted to Kaplan–Meier data, we had opted to use the mathematically best-fitting curves so long as they were clinically plausible and were a visual good fit. In some cases, these differ from the curves used by ICER. The difference in survival estimation did not have a significant impact on the model results. In calculating the probability of transitioning to death, the ICER model used observed deaths for the clinical trial period (short-term model period) and then applied survival curve-based mortality to the extrapolated period of the model for patients in all health states; our model did this for D state patients only. Our model used cycle lengths of six months for the first three years, and annual cycles thereafter; the ICER model used monthly cycles throughout the duration of the model. In calculating the proportion of patients who achieved motor milestones, we conservatively assumed that all milestones occurred at the end of each cycle and so account for these transitions in the next full cycle. As a result, the QALYs for each arm may be underestimated and costs overestimated.
Results
The base case results for the revised model are presented in . SMA type 1 patients enter the non-sitting (‘D’) state of the model before the age of 6 months. Undiscounted life years predicted by our updated model per patient in the onasemnogene abeparvovec arm were 37.60 life-years compared to 12.10 life-years for nusinersen and 7.27 life-years for BSC (20.09, 9.06, and 5.91 life-years, respectively, when discounted at 3%). Median survival was 35.0 years in the onasemnogene abeparvovec arm compared to 16.0 years and 3.0 years for nusinersen and BSC, respectively. When utility weightings were applied, discounted QALYs per patient in the onasemnogene abeparvovec arm were 13.33 compared to 2.85 for nusinersen and 1.15 for BSC. These results broadly align with ICER’s model, which reported 13.46 QALYs for onasemnogene abeparvovec in its analysis against nusinersen, 3.24 QALYs for nusinersen and 0.46 QALYs for BSC.
Table 3. Comparison of results from old model, new model and ICER model
Table 4. Results of key scenario analyses in the updated model – Onasemnogene abeparvovec vs Nusinersen
Our updated model estimated mean lifetime per-patient cost to a US commercial payer (when discounted at 3%) to be 3,930,879 USD for onasemnogene abeparvovec (using the list price of 2,125,000 USD), 4,602,692 USD for nusinersen, and 1,961,710 USD for BSC. These costs were primarily driven by the high costs of therapy in the onasemnogene abeparvovec and nusinersen arms and the high cost of medical treatment in the BSC arm. The ICER model also predicted that the lifetime cost of onasemnogene abeparvovec would be lower than that of nusinersen. Costs predicted by the ICER model were 3,657,000 USD for onasemnogene abeparvovec using the 2,000,000 USD placeholder price (similar to 3,782,000 USD for the list price), 3,884,000 USD for nusinersen, and 789,000 USD for BSC.
At the list price of 2,125,000 USD for onasemnogene abeparvovec, the incremental cost per QALY gained was 161,648 USD compared to BSC, which is lower than the result estimated by ICER (243,000 USD), due to ICER’s milestone assumptions. When compared to nusinersen, our updated model predicts that onasemnogene abeparvovec will be dominant (less costly and more effective). In contrast, ICER predicted that the cost per QALY gained for onasemnogene abeparvovec compared to nusinersen would be 139,000 USD (this analysis includes cost of sequential nusinersen therapy). ICER’s estimate falls below traditional willingness to pay thresholds in the US, while the results predicted by our updated model suggest that onasemnogene abeparvovec may not be cost-effective at the current list price against BSC, but is dominant against nusinersen. With two disease-modifying treatments now available for SMA type 1 in the US, we consider that BSC is not an appropriate comparator for decision-makers (as BSC is not a viable or ethical alternative to therapy).
Discussion
The results of the revised model align with the results of the ICER model with some, but not all, outcomes. Both models predict similar discounted life-years for onasemnogene abeparvovec (20.09 in the updated model; 19.76 in the ICER model comparing onasemnogene abeparvovec to nusinersen with no loss of milestones). Our revised model predicts more discounted life-years for nusinersen compared to the ICER model (9.06 vs 7.64 life-years), as well as estimating more discounted life-years for BSC (5.91 life-years compared to 2.40 life-years). This is because our model uses data from tracheostomized patients (who survive longer) when estimating survival in the Permanent Assisted Ventilation (PAV) state, compared to the ICER model which uses data from patients reliant on non-invasive ventilation only (who have limited survival). Compared to our previous model, estimated survival for nusinersen has also increased (from 7.11 life-years to 9.06 life-years); we surmise that this is because of the revised data source to estimate the number of patients who transition to the Permanent Assisted Ventilation state (from NeuroNext to ENDEAR sham control). In the updated CUA, more patients transition to the PAV state (with longer survival) than in the previous version of the model.
The updated model predicts 13.33 QALYs for onasemnogene abeparvovec, a number that closely aligns with the 13.46 QALYs predicted by the ICER model in the scenario comparing onasemnogene abeparvovec to nusinersen. The updated model predicts 2.85 QALYs for nusinersen, compared to 3.24 QALYs predicted by the ICER model. In effect, the updated model predicts longer survival for nusinersen patients than the ICER model, but fewer QALYs. Overall, our updated model applies an average utility weighting of 0.31 to nusinersen patients (2.85 QALYs divided by 9.06 life-years) compared to the average 0.42 utility weighting used by ICER (3.24 QALYs divided by 7.64 life-years). While the utility scores applied to each health state are the same across both models, the difference arises because ICER’s estimates of the proportion of nusinersen patients who sit are higher (because they include patients who did not attend follow-up visits) and because our model only transitions patients into higher milestone groups at the start of the next full 6-month cycle, which will underestimate the amount of time patients spend in the sitting and walking states (with higher utility scores) for both treatment arms compared to the ICER model (which transitions patients every month). Additionally, in comparison to our previous model, the updated CUA predicts fewer QALYs for all arms because the utility scores used by ICER for the Permanent Ventilation and Non-sitting health states are much lower than the scores we originally used.
The updated model estimates that the average lifetime cost of onasemnogene abeparvovec therapy is 3,930,879 USD compared to the ICER estimate of 3,657,000 USD for onasemnogene abeparvovec at the placeholder price of 2,000,000 USD (this would be 3,782,000 USD using the 2,125,000 USD list price). For nusinersen, our updated model estimates the average lifetime cost will be 4,602,692 USD compared to ICER’s estimates of (base case), 3,884,000 USD for nusinersen. This represents a substantial change compared to our previous model, which predicted much higher costs associated with nusinersen treatment (largely driven by the reported hospital markup applied in some instances). For BSC, our updated model predicts lifetime costs of 1,961,710 USD compared to ICER’s prediction of 789,000. USD The remaining differences in estimated lifetime costs between our updated model and the ICER model are attributable to the different life-years predicted by each model (as the average cost per life-year is similar between the two models), as well as the previously mentioned differences in timing of health state transitions, as the cost inputs are close to equivalent.
The updated model predicts that average total lifetime cost of onasemnogene abeparvovec treatment will be lower than the cost of nusinersen treatment, with additional benefits; hence, onasemnogene abeparvovec is dominant against nusinersen. That onasemnogene abeparvovec is cost-effective against nusinersen according to traditionally used cost-effectiveness thresholds is consistent with the conclusion of the ICER analysis (a cost per QALY of 139,000 USD when comparing onasemnogene abeparvovec directly to nusinersen); however, this naïve comparison ‘scenario’ incorporates additional cost of nusinersen treatment for some onasemnogene abeparvovec patients. As an alternative analysis using the published ICER results, comparison of the ICER-modeled lifetime cost of onasemnogene abeparvovec to the ICER-modeled cost of nusinersen (3,657,000 compared to 3,884,000 USD), and the ICER-modeled QALYs of onasemnogene abeparvovec compared to those of nusinersen (12.23 compared to 3.24), results in a negative cost per QALY (i.e., the cost per QALY of onasemnogene abeparvovec would similarly be considered ‘dominant’ compared to nusinersen).
In our exploratory analysis of presymptomatic patients, we predicted that onasemnogene abeparvovec would deliver 22.83 discounted life-years and nusinersen would deliver 21.57 life-years. Quality-adjusted survival predicted by the model was 16.47 QALYs for onasemnogene abeparvovec and 14.87 QALYs for nusinersen. This was slightly below the values estimated by ICER (both arms with 26.58 LYs and 21.94 QALYs). We expect the difference is due to the timing of health state transitions as our model only transitions patients at the start of each new six-month cycle. In our exploratory presymptomatic model, the lifetime cost of nusinersen substantially exceeds onasemnogene abeparvovec (10,589,128 vs 3,803,121 USD), so onasemnogene abeparvovec dominates in this population. The ICER model predicted similar outcomes: lifetime costs for nusinersen of 11,929,000 USD compared to 3,264,000 USD for onasemnogene abeparvovec, for 21.94 QALYs with either treatment.
In the updated model, we also added an exploratory analysis from a modified societal perspective, to reflect the impacts of treatment outside of the health system. In this analysis, we mirrored the ICER estimates, adding values for economic inputs listed in ICER report: non-medical costs (e.g., moving or modifying the home and purchasing or modifying a vehicle), lost household income arising from caring for SMA patients, and patient productivity gains (for SMA patients who may be able to work during adulthood). The societal perspective also incorporates caregiver disutilities, to capture effects on caregiver quality of life. Input values for this scenario are included in . In this analysis, the total lifetime cost for each treatment option is higher (as this analysis includes additional costs outside of the health system, which are not offset by the gain in labor market earnings): 4,328,330 USD for onasemnogene abeparvovec and 4,874,280 USD for nusinersen. In terms of benefits, life-years remain unchanged but QALYs are substantially adjusted due to the use of negative utility weightings for all health states except for walking (QALYs reduced to 10.02 for onasemnogene abeparvovec and −0.13 for nusinersen). Overall, this analysis supports the conclusion that onasemnogene abeparvovec is cost-effective compared to nusinersen in symptomatic SMA type 1 from both the societal and payer perspective.
An important limitation of our model is that we are unable to follow patients for the full duration of the expected benefit (up to a lifetime), leading to considerable uncertainty around the future survival improvement of all treated patients. Like the ICER model, we used scenario and sensitivity analyses to explore the impact of more pessimistic assumptions. In a scenario assuming a waning of effect for onasemnogene abeparvovec patients beyond 25 years (if patients began to lose milestones around adulthood), onasemnogene abeparvovec generates 10.25 QALYs compared to nusinersen’s 2.85, at a lower cost overall. In highly pessimistic scenarios further reducing the estimated effect of gene therapy, QALYs reduce but still remain higher overall compared to nusinersen (5.66 and 7.54 QALYs for onasemnogene abeparvovec-treated patients assuming duration of effect of 10 and 15 years, respectively), while lifetime costs remain lower than those of nusinersen. This analysis illustrates the value of onasemnogene abeparvovec as a ‘one-off’ treatment with clinically meaningful benefits compared to chronic therapy in the symptomatic SMA type 1 population, even with uncertainty around the future longevity of those benefits.
Lifetime costs for onasemnogene abeparvovec patients in that scenario are 3,788,187 USD and hence onasemnogene abeparvovec is still dominant against nusinersen in that scenario. Long-term follow-up of the efficacy of onasemnogene abeparvovec will be important to demonstrate the durability of benefits for patients. There is reason to consider that the benefits are likely to be permanent as motor neurons are long-lived; one-time administration of gene therapy is thought to be sufficient for lifetime episomal transgene expression in the cell if delivered before motor neuron loss occurs [Citation7].
Another important limitation is the lack of direct comparison between onasemnogene abeparvovec and nusinersen. However, in the case of SMA trials, the relative homogeneity of the enrolled patient groups (all study participants had two copies of the SMN2 gene, and mean age at disease onset was 1.8 months, 2.2 months, and 1.4 months for nusinersen, sham control, and onasemnogene abeparvovec, respectively), and consistent use of objective clinical endpoints (gross motor milestone achievement; need for permanent ventilation) assist in making these comparisons. A 2019 indirect treatment comparison concluded that onasemnogene abeparvovec may have an efficacy advantage relative to nusinersen for overall survival, independence from permanent assisted ventilation, motor function, and motor milestones [Citation26].
Further efficacy studies are underway to evaluate onasemnogene abeparvovec, including open-label, single-arm phase 3 trials in infants with SMA type 1 younger than six months of age, with one or two copies of the SMN2 gene in both the USA (NCT03306277 [STR1VE], and the European Union (NCT03461289 [STR1VE-EU]). The US STR1VE trial has recently concluded (although final data are not available as of this writing), and the STR1VE-EU trial has completed enrollment. In addition, a phase 3 global study (NCT03505099 [SPR1NT]) evaluating the efficacy of onasemnogene abeparvovec in presymptomatic neonatal patients with SMA (age six weeks or less at dosing) with multiple SMN2 copies (two and three copies) is currently ongoing. Notably, one patient in the STR1VE-US trial died at age 7.8 months due to respiratory failure, and one patient’s death has been reported in the STR1VE-EU trial following onset of respiratory distress and hypoxic-ischemic encephalopathy; both deaths were considered unrelated to treatment by the investigator. The updated model partially accounts for this by a scenario where overall survival is 95% for the clinical trial period. In this scenario, the life-years, QALYs and lifetime costs of onasemnogene abeparvovec are reduced; onasemnogene abeparvovec remains dominant against nusinersen.
Recent approvals of potentially curative gene therapies for previously fatal childhood diseases have precipitated debate on whether CUA methods are adequate to capture the true value of these therapies to society, without binding payers to unsustainable prices, especially as more gene therapies are approved [Citation27]. This updated CUA analysis, and the preceding ICER report, suggest that, in the case of fatal, devastating diseases such as SMA, the value of restoring the potential for a full life is clear and the challenge becomes ensuring that the ‘right’ patients are treated at the ‘right’ time – that is, identifying patients who will likely benefit and restoring healthy gene function in time to halt disease progression before life-threatening, irreversible damage has occurred. Newborn screening and early intervention in children with genetically confirmed SMA represent the best means of identifying and treating the ‘right’ patients, and will thus deliver substantial value to the health system, and improve the lives of children and families impacted by this disease.
Conclusion
At the list price of 2,125,000 USD in the US, onasemnogene abeparvovec is cost-effective compared to nusinersen for symptomatic SMA type 1 patients treated before two years of age and is close to being cost-effective compared to BSC for presymptomatic SMA type 1 when traditional cost-effectiveness thresholds are used. In presymptomatic patients, scenario analyses show that single-dose onasemnogene abeparvovec treatment may be as effective as nusinersen and substantially less costly. While there are some key differences between our updated model and the ICER model, the two models corroborate each other by producing similar results.
Acknowledgments
The authors acknowledge Ramesh Arjunji, formerly of Novartis Gene Therapies, for substantial contributions to the cost-utility model and for critical evaluation of early manuscript drafts. The authors would like to thank Richa Chhabra, an employee of Novartis Healthcare Pvt. Ltd., Hyderabad for formatting the manuscript as per journal guidelines and supporting the submission of the manuscript.
Disclosure statement
Rebecca Dean, Ivar Jensen, Phil Cyr and Beckley Miller are employees of PRECISIONheor and PRECISIONheor received funding for the analysis from Novartis Gene Therapies. Benit Maru and Thomas Wiesner are employees of SSI Strategy, who were contracted to support Novartis Gene Therapies. Douglas M. Sproule, Douglas E. Feltner are former employees of Novartis Gene Therapies. Omar Dabbous, Matthias Bischof and Walter Toro are employees of Novartis Gene Therapies. Daniel C. Malone is a consultant to Novartis.
Additional information
Funding
References
- ZOLGENSMA (Onasemnogene abeparvovec) PI. In: Administration UFaD, ed2019.
- Mercuri E, Finkel RS, Muntoni F, et al. Diagnosis and management of spinal muscular atrophy: part 1: recommendations for diagnosis, rehabilitation, orthopedic and nutritional care. Neuromuscul Disord. 2018;28(2):103–12.
- Lally C, Jones C, Farwell W, et al. Indirect estimation of the prevalence of spinal muscular atrophy Type I, II, and III in the USA. Orphanet J Rare Dis. 2017;12(1):175.
- De Sanctis R, Pane M, Coratti G, et al. Clinical phenotypes and trajectories of disease progression in type 1 spinal muscular atrophy. Neuromuscul Disord. 2018;28(1):24–28.
- Finkel RS, McDermott MP, Kaufmann P, et al. Observational study of spinal muscular atrophy type I and implications for clinical trials. Neurology. 2014;83(9):810–817.
- Services. DoHaH. Recommended Uniform Screening Panel (2018, July). In: Services. DoHaH, ed2018.
- Al-Zaidy SA, Mendell JR. From clinical trials to clinical practice: practical considerations for gene replacement therapy in SMA type 1. Pediatr Neurol. 2019;100:3–11.
- Mendell JR, Al-Zaidy S, Shell R, et al. Single-dose gene-replacement therapy for spinal muscular atrophy. N Engl J Med. 2017;377(18):1713–1722.
- Al-Zaidy S, Pickard AS, Kotha K, et al. Health outcomes in spinal muscular atrophy type 1 following AVXS-101 gene replacement therapy. Pediatr Pulmonol. 2019;54(2):179–185.
- Malone DC, Dean R, Arjunji R, et al. Cost-effectiveness analysis of using onasemnogene abeparvocec (AVXS-101) in spinal muscular atrophy type 1 patients. J Mark Access Health Policy. 2019;7(1):1601484.
- Zerres K, Rudnik-Schöneborn S, Forrest E, et al. A collaborative study on the natural history of childhood and juvenile onset proximal spinal muscular atrophy (type II and III SMA): 569 patients. J Neurol Sci. 1997;146(1):67–72.
- Shieh PB, Gu T, Chen E, et al. Treatment patterns and cost of care among patients with Spinal Muscular Atrophy. Cure SMA Annual Meeting; June 29-July 1, 2017, 2017; Orlando, FL.
- Gregoretti C, Ottonello G, Chiarini Testa MB, et al. Survival of patients with spinal muscular atrophy type 1. Pediatrics. 2013;131(5):e1509–1514.
- (ICER) IfCaER. Spinraza and Zolgensma for Spinal Muscular Atrophy: Effectiveness and Value. Final Evidence Report. April 3, 2019 (Updated May 24, 2019) 2019.
- Review IIfCaE. Modifications to the ICER value assessment framework for treatments for ultra-rare diseases. November 2017 2017.
- Castro D, Farrar M, Finkel R, et al. Longer-term assessment of the safety and efficacy of nusinersen for the treatment of infantile-onset Spinal Muscular Atrophy (SMA): an interim analysis of the SHINE study. American academy of neurology. Los Angeles, CA;2018. April 21–27.
- Finkel RS, Mercuri E, Darras BT, et al. Nusinersen versus Sham control in infantile-onset spinal muscular atrophy. N Engl J Med. 2017;377(18):1723–1732.
- Kolb SJ, Coffey CS, Yankey JW, et al. Natural history of infantile-onset spinal muscular atrophy. Ann Neurol. 2017;82(6):883–891.
- Thompson R, Vaidya S, Teynor M The utility of different approaches to developing health utilities data in childhood rare diseases – a case study in Spinal Muscular Atrophy (SMA). ISPOR 20TH Annual European Congress 4–8 November 2017; 2017; Glasgow, Scotland.
- Tappenden P, Hamilton J, Kaltenthaler E, et al. Nusinersen for treating spinal muscular atrophy: a single technology appraisal. School of Health and Related Research (ScHARR); 2018.
- Noyes J, Godfrey C, Beecham J. Resource use and service costs for ventilator-dependent children and young people in the UK. Health Soc Care Community. 2006;14(6):508–522.
- Lowes LP, Alfano LN, Arnold WD, et al. Impact of age and motor function in a phase 1/2A study of infants with SMA type 1 receiving single-dose gene replacement therapy. Pediatr Neurol. 2019;98:39–45.
- Van Den Broek BTA, Page K, Paviglianiti A, et al. Early and late outcomes after cord blood transplantation for pediatric patients with inherited leukodystrophies. Blood Adv. 2018;2(1):49–60.
- Finkel RS, Mercuri E, Meyer OH, et al. Diagnosis and management of spinal muscular atrophy: part 2: pulmonary and acute care; medications, supplements and immunizations; other organ systems; and ethics. Neuromuscul Disord. 2018;28(3):197–207.
- Bach JR, Gupta K, Reyna M, et al. Spinal muscular atrophy type 1: prolongation of survival by noninvasive respiratory aids. Pediatr Asthma Allergy Immunol. 2009;22(4):151–161.
- Dabbous O, Maru B, Jansen JP, et al. Survival, motor function, and motor milestones: comparison of AVXS-101 relative to nusinersen for the treatment of infants with spinal muscular atrophy type 1. Adv Ther. 2019;36(5):1164–1176.
- Pearson SD, Ollendorf DA, Chapman R. New Cost-Effectiveness methods to determine Value-Based Prices for potential cures: what are the options? Value Health. 2019;22(6):656–660.
- Mendell JR, Al-Zaidy S, Shell R, et al. AVXS-101 Phase 1 gene replacement therapy clinical trial in SMA type 1: continued event-free survival and achievement of developmental milestones. 22nd Annual SMA Researcher Meeting; June 14–16, 2018, 2018; Dallas, TX.
- Database USM. Human mortality database. 2017; https://usa.mortality.org/
- The Lewis Group Inc. Cost of amyotrophic lateral sclerosis, muscular dystrophy, and spinal muscular atrophy in the USA. 2012; https://www.mda.org/sites/default/files/Cost_Illness_Report_0.pdf
