ABSTRACT
Background: Evidence suggests that triple therapy for patients with chronic obstructive pulmonary disease (COPD) is being used in a broader range of patients than recommended by guidelines, which may have health and cost implications.
Objective: To explore the relationship between national health technology assessment (HTA) agency appraisals and market penetration of two fixed-dose combination (FDC) triple therapies.
Study design: HTAs from Q3 2017 to Q1 2020 from 10 countries were evaluated.
Intervention: Glycopyrronium bromide/formoterol fumarate/beclomethasone (Trimbow®) and umeclidinium/vilanterol/fluticasone furoate (Trelegy™ Ellipta®).
Main outcome measure: HTA restrictions and prescribing rates (days of therapy).
Results: Seven countries (70%) imposed restrictions on use including prescription only for patients stable on free-combination triple therapy or not controlled on dual therapy, requirement of a specialist prescription or therapeutic plan, prescription only for patients with severe COPD, and use as second-line therapy or later. In general, countries that have imposed restrictions on the use of FDC triple therapies have seen a lower than average uptake.
Conclusion: Payer guidance on prescribing FDC triple therapy may potentially support more appropriate prescribing in line with clinical guidelines. It is important for payers to consider which restrictions would ensure the most efficient use of scarce resources.
Introduction
The 2020 Global Initiative for Chronic Obstructive Lung Disease (GOLD) strategy document recommends that triple therapy, with a long-acting β2 agonist (LABA), a long-acting muscarinic antagonist (LAMA), and inhaled corticosteroids (ICS), be considered for a select group of patients with chronic obstructive pulmonary disease (COPD) who continue to exacerbate despite treatment with dual therapy [Citation1]. Dual therapy with a LAMA/LABA continues to be the mainstay of long-term COPD maintenance treatment [Citation1,Citation2].
There is a general discordance between the recommendations in COPD guidelines and real-life clinical practice [Citation3]. Real-world evidence suggests that triple therapy, both free combination (i.e., separate inhalers) and fixed-dose combination (FDC), is being frequently prescribed to patients outside recommendations by GOLD and country-specific guidelines [Citation4–15]. A number of studies have recently assessed the efficacy and safety of triple therapy [Citation16–19]. This evidence suggests that triple therapy has a role in patients who have frequent exacerbations and high blood eosinophil counts. Number-needed-to-treat analyses confirm this and demonstrate that the largest effect is in reducing the number of exacerbations in patients having multiple events [Citation18]. In general, guidelines do not support the use of triple therapy in a broad COPD population, but rather as a step-up from dual therapy in a select group of symptomatic patients, with moderate-to-severe exacerbation history, despite treatment with bronchodilator therapy. In addition, there is clear guidance that ICS should be stopped if there are adverse effects or reported lack of efficacy while on triple therapy [Citation1]. Therefore, in addition to the benefits of triple therapy, overutilisation can increase the risk of pneumonia [Citation20] and other adverse effects associated with ICS use [Citation21]. In addition, real-world evidence suggests that inappropriate use of triple therapy can increase drug and healthcare resource use [Citation11].
In the last three years, two FDC triple therapies have been granted marketing authorisation: glycopyrronium bromide/formoterol fumarate/beclomethasone (Trimbow®) and umeclidinium/vilanterol/fluticasone furoate (Trelegy™ Ellipta®). The introduction of these new FDC triple therapies may increase uptake that is not in accordance with guideline recommendations, which may increase avoidable adverse effects and healthcare costs. To date, there are few studies evaluating the uptake of FDC triple therapies and none that assess uptake versus payer recommendations across different countries. We identified and described national health technology assessment (HTA) agency appraisals of two FDC triple therapies, analysed the market penetration of these FDC triple therapies relative to alternative inhaled therapies, and explored the relationship between national HTA agency appraisals and market penetration in seven European countries, Australia, Canada, and the US. This information can help provide additional data to help payers advise or mandate on the most appropriate use of these products.
Methods
National HTA evaluations were retrieved from respective agency sites for the seven European countries, Australia, and Canada (). For the US, where there is no centralised HTA procedure, the prescribing information from the Food and Drug Administration (FDA) was reviewed. HTA recommendations were summarised and categorised as ‘no restrictions’, ‘restrictions’, or ‘no assessment’. Where a country had imposed restrictions, the type of restriction was described.
Table 1. HTA and national agencies
The number of doses per quarter of a year for each drug within the LAMA, LAMA/LABA, LABA/ICS, and LAMA/LABA/ICS class was extracted from IQVIA MIDAS® international data (Q3 2017 to Q1 2020). The data on doses were standardised by calculating days of therapy (DOT) for each drug within each class by dividing the number of doses by average daily dose (AVDD). The AVDDs that were used in this analysis were either sourced from IQVIA MIDAS® international data, the World Health Organization (WHO) [Citation20], or, in a limited number of cases, directly from the product summary of product characteristics. Where there were multiple forms for the same product with different AVDDs (e.g., Seretide is available as an inhalation powder and inhalation aerosol), the higher of the AVDDs were used. There is limited information in the IQVIA dataset on the product form (e.g., powder or aerosol).
The time from marketing authorisation to first prescription was assessed and compared to the presence of HTA restrictions. Market share of Trelegy™ and Trimbow® was determined by calculating DOT as a percentage of total market share for each quarter and for each country. Total market share was defined as the total DOT for LAMA, LABA/ICS, LAMA/LABA, and LAMA/LABA/ICS combined.
Market share of Trelegy™ and Trimbow® was then averaged across all countries over time, anchoring the first quarter for each country as the quarter in which doses were identified to have first been prescribed. The market share for Trelegy™ and Trimbow® for each individual country was then compared to this average, quantifying how far above or below a country was from this value over time. The distance each country was away from the average market share was calculated using data from the eighth quarter of prescribing or, if not available, the last quarter a value was reported. Countries were categorised into four groups depending on first, whether there were imposed HTA restrictions on FDC triple therapy use and second, whether they lay above or below the average market share (calculated quarterly as a percentage of total market share).
A number of sensitivity analyses were carried out. First, in order to assess the impact of using the higher AVDD where there were multiple forms of the same product, we estimated the market share of Trelegy™ and Trimbow® as a % of overall market share using the lower of the AVDDs. Second, the assessment of average uptake of Trelegy™ and Trimbow® was by yearly quarters; therefore, comparisons between countries could be biased towards those countries where prescribing started at the beginning of the quarter. To assess the impact of this, we carried out a sensitivity analysis where the first quarter was defined as a percentage market share for Trelegy™ and Trimbow® of over 0.10% of the total market. This definition therefore removes data where prescribing was very low due to the introduction of Trelegy™ and Trimbow® at the end of a quarter.
Results
The majority of countries imposed a form of restriction on prescribing (). These included Australia, Canada, France, Italy, the Netherlands, and Spain. In the UK, Scotland imposed restrictions, but England did not complete an HTA as triple therapy is made up of molecules already licensed for use. Sweden did not impose any restrictions, and there is no centralised national HTA procedure in the US. In Germany, the G-BA only appraised Trelegy™ and determined that the magnitude of the additional benefit was ‘not proven’ for Trelegy™ versus free combination triple therapy. This was considered a restriction for the purposes of this analysis. The G-BA did not appraise Trimbow®.
Table 2. Summary of HTAs
The forms of restrictions varied between countries and included access to only patients stable on free combination triple (the Netherlands, Spain), requirement of a specialist prescription (France, Italy) or a therapeutic plan (Italy), access only to patients with severe disease (Australia, France) or to patients not controlled on dual therapy (Canada), and no use as initial therapy (Australia, Canada). Payers carried out separate appraisals for Trelegy™ and Trimbow®, and restrictions were consistent between the two FDC triple therapies.
The time from marketing authorisation to first prescription was, in general, longer for those markets implementing an HTA and imposing restrictions on use (). Note that in some countries access to new medicines may be possible prior to the HTA (e.g., Germany).
Figure 1. Time from marketing authorisation to first sales: (a) Trelegy™ and (b) Trimbow®
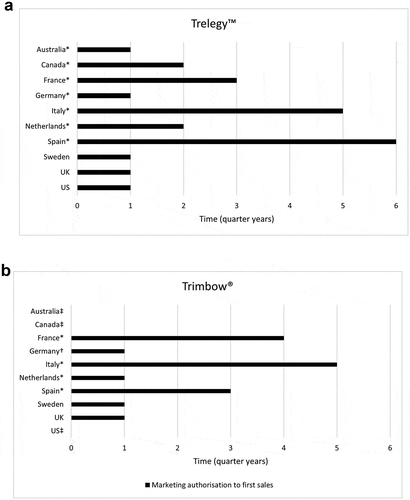
For Trelegy™, Australia, UK, and the US had above average uptake. Canada, France, Germany, the Netherlands, and Sweden saw a lower than average uptake. Italy and Spain, with limited data due to delayed introduction of Trelegy™, demonstrated slightly below average uptake (). For Trimbow®, there was a similar pattern; however, volume market share showed much less variation between countries than for Trelegy™ ().
Figure 2. Volume of market share: (a) Trelegy™ and (b) Trimbow®
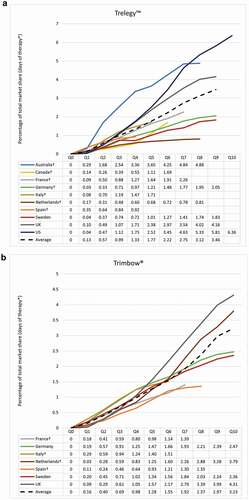
In general, countries that have imposed HTA or guideline restrictions on the use of FDC triple therapy have seen a lower uptake (). For Trelegy™, the two exceptions were Australia and Sweden. Sweden had lower than average uptake despite no restrictions and Australia had higher than average uptake despite restrictions. For Trimbow®, again there is much less variation; however, the Netherlands had marginally higher than average uptake of Trimbow® despite restrictions and Sweden had marginally lower than average uptake despite no restrictions. This was also the case in Germany where no HTA assessment of Trimbow® was identified. For both Trelegy™ and Trimbow® combined, of the countries where payers had imposed restrictions, over 70% saw a lower than average uptake.
Figure 3. Assessment of uptake of FDC triple therapy by demonstrating distance away from the average percentage of total market share and the relationship to HTA restrictions: (a) Trelegy™ and (b) Trimbow®
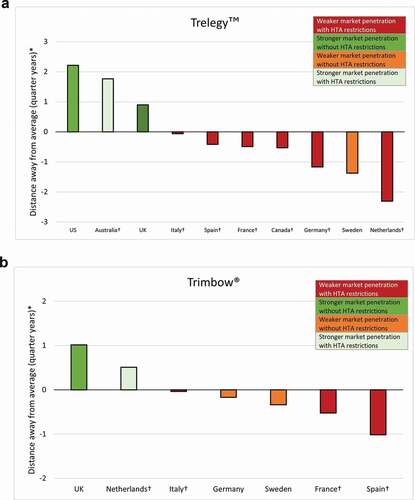
The sensitivity analysis confirmed the robustness of the results (). The only change in categorisation was Italy, which changed from a base-case that lay marginally below the average percentage of total market share to marginally above it in the sensitivity analyses.
Table 3. Sensitivity analyses of distance away from the average percentage of total market share and the relationship to HTA restrictions for Trelegy™ and Trimbow®
Discussion
HTAs refer to the systematic evaluation of the impact of a health intervention, taking into account an assessment of social, economic, organisational, and/or ethical issues. The primary purpose of HTA is to inform policy decision-making on healthcare spending to ensure efficient use of scarce resources. There are, however, wide variations in the methods used by HTA agencies that lead to differences in recommendations. Furthermore, there are differences whether recommendations are mandatory and in how FDCs of substances already approved for use are appraised by HTA agencies.
Our study demonstrates that in the majority of countries, HTA agencies have imposed a form of restriction on prescribing FDC triple therapy, and exploratory analysis shows that, in general, where there is national HTA guidance in place, this can have an impact on the prescribing of FDC triple therapy. Payers who have taken this additional step to support more appropriate prescribing introduced a range of measures to control use. These ranged from restricting access to only patients stable on free combination triple (the Netherlands, Spain), to only patients with severe disease (Australia, France), or to only in patients not controlled on dual therapy (Canada). Others included the requirement of a specialist prescription (France, Italy) or a therapeutic plan (Italy). Such restrictions are in line with international COPD guidelines [Citation1] in most cases, except where restrictions were imposed in relation to severity of disease (Australia, France). GOLD guidelines no longer use airflow limitation to drive treatment choices; instead, recommendations are based on symptoms and history of exacerbations [Citation1].
There are a number of other factors, aside from HTA recommendations, that may influence market uptake of FDC triple therapy and explain why countries can have a lower than average uptake despite a lack of restrictions, and other countries can have a higher than average uptake despite restrictions. These will vary by country and could include a promotion of, and growth in, the overall triple therapy market [Citation4,Citation15]; an historic high use of ICS-based therapies and lack of adherence to guidelines [Citation6–15]; a preference by the patient or prescriber for a specific type of inhaler (e.g., preference for a metered dose inhaler, like Trimbow®, over dry powder inhalers, like the majority of LAMA/LABAs [Citation22]); a lack of spirometry testing to determine disease severity [Citation23]; and off-label use in patients with asthma or asthmatic symptoms [Citation23].
In most countries, there is evidence of the growth and overuse of ICS-based therapies including triples, which is not in accordance with clinical guidelines. Despite guidelines recommending reserving the use of triple therapy for patients in GOLD group D (severe symptoms with ≥2 moderate/severe exacerbations in the past year [or ≥1 leading to hospital admission]) who continue to experience exacerbations on dual therapy, real-world data show that these guidelines are not being followed in actual clinical practice. Evidence across Europe and the US demonstrates that up to three-quarters of patients with less severe COPD, defined by exacerbation history and/or symptoms, are receiving triple therapy, contrary to treatment recommendations [Citation4,Citation8–12,Citation14,Citation15]. In Australia, 24% of patients with GP-classified mild COPD and 43% of patients with moderate COPD were on triple therapy [Citation13].
In addition, patients with newly diagnosed COPD are also commonly prescribed triple therapy which is not recommended in clinical guidelines [Citation1]. In the UK, two separate population-based studies showed that 9% [Citation6] and 18% [Citation9] of patients with less severe COPD, defined by exacerbation history and symptoms, initiating maintenance therapy were prescribed triple therapy. In a Canadian database study, over two-thirds (71%) of patients with newly diagnosed COPD initiated triple therapy [Citation7].
There are a number of limitations with this study. First, we only included FDC triple therapy. It is not known from these data the extent to which FDC uptake is due to switching from free-dose combinations or patients naive to triple therapy. Second, there are a limited number of data points for some countries (Trelegy™: 6 out of 10 countries have eight data points; Trimbow®: five out of seven countries have eight data points). Third, as prescribing data do not contain information on product form (e.g., powder or aerosol), it was not possible to determine the exact AVDD for the LABA/ICS class. However, a sensitivity analysis was conducted, which confirmed the overall conclusions. Fourth, assessment of average uptake of FDCs by yearly quarters and comparisons between countries may be biased towards those countries whose sales started at the beginning of the quarter. Again, a sensitivity analysis confirmed that this has little impact on the overall results. Last, only national HTA guidelines are reported. In Spain and Italy, where there is some devolution of HTA responsibilities to autonomous regions, we examined local guidelines in a sample of regions which aligned with national guidelines, where available.
Set against a background of historical overuse of ICS-based regimens, the lack of appropriate control on the use of triple FDCs could exacerbate a situation that has both health and economic implications. Patients could be exposed to unnecessary adverse effects [Citation19] and healthcare costs could escalate [Citation11]. Our study demonstrated that, in general, where there is HTA or appropriate payer guidance in place, this may have an impact on the use of FDC triple therapy and support more appropriate use. It is essential for payers to assess the range of factors that impact uptake of new FDCs in the field of COPD, including mechanisms aimed at supporting prescribing consistent with guidelines and maximising efficient use of scarce healthcare resources.
Contributors
JC and SL designed the research. JL, ZM, and SL conducted the research. JL drafted the manuscript. SL supervised the writing. JC, CB, JL, ZM, JHP, and SL contributed to the data interpretation and revised each draft for important intellectual content. All authors read and approved the final manuscript. JC had primary responsibility for the final content and is the guarantor. The corresponding author (SL) attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Disclosure of potential conflicts of interest
JL, ZM and SL are employees of Maverex Limited, which received funding from Boehringer Ingelheim to conduct the study. JC and JHP are employees of Boehringer Ingelheim. CB has no potential conflicts of interest to disclose.
Data availability statement
The data that support the findings of this study are available from IQVIA. Restrictions apply to the availability of these data, which were used under license for this study. Data are available from the first author, JC, with the permission of IQVIA.
Additional information
Funding
References
- GOLD. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease [Internet]. 2020 [cited 2020 Apr 16]. Available from: https://goldcopd.org/wp-content/uploads/2019/12/GOLD-2020-FINAL-ver1.2-03Dec19_WMV.pdf
- Mammen MJ, Pai V, Aaron SD, et al. Dual LABA/LAMA therapy versus LABA or LAMA monotherapy for COPD: a systematic review and meta-analysis in support of the American Thoracic Society Clinical Practice Guideline. Ann Am Thorac Soc. 2020;19(9):1133–9.
- Bloom CI, Elkin SL, Quint JK. Changes in COPD inhaler prescriptions in the UK, 2000 to 2016. Int J Chron Obstruct Pulmon Dis. 2019;14:279–287.
- Adir Y, Horner A, De Vries GJ, et al. Treatment patterns in stable COPD patients – results from a large observational European study (SPACE). Eur Respir J. 2019;54(suppl 63):PA2509.
- Barrecheguren M, Monteagudo M, Ferrer J, et al. Treatment patterns in COPD patients newly diagnosed in primary care. A population-based study. Respir Med. 2016;111:47–53.
- Chalmers JD, Poole C, Webster S, et al. Assessing the healthcare resource use associated with inappropriate prescribing of inhaled corticosteroids for people with chronic obstructive pulmonary disease (COPD) in GOLD groups A or B: an observational study using the Clinical Practice Research Datalink (CPRD). Respir Res. 2018;19(1):63.
- Dhalwani N, Cabrera C, Booth A, et al. Characteristics and treatment patterns of newly diagnosed COPD patients in Canada. Eur Respir J. 2019;54(suppl 63):PA3385.
- Graf J, Jörres RA, Lucke T, et al. Medical treatment of COPD. Dtsch Arztebl Int. 2018;155(37):599–605.
- Halpin DMG, de Jong HJI, Carter V, et al. Distribution, temporal stability and appropriateness of therapy of patients with COPD in the UK in relation to GOLD 2019. EClinicalMedicine. 2019;14:32–41.
- Lopez-Campos JL, Navarrete BA, Soriano JB, et al. Determinants of medical prescriptions for COPD care: an analysis of the EPOCONSUL clinical audit. Int J Chron Obstruct Pulmon Dis. 2018;13:2279–2288.
- Palli SR, Buikema AR, DuCharme M, et al. Costs, exacerbations and pneumonia after initiating combination tiotropium olodaterol versus triple therapy for chronic obstructive pulmonary disease. J Comp Eff Res. 2019;8(15):1299–1316.
- Palmiotti GA, Lacedonia D, Liotino V, et al. Adherence to GOLD guidelines in real-life COPD management in the Puglia region of Italy. Int J Chron Obstruct Pulmon Dis. 2018;13:2455–2462.
- Reddel HK, Valenti L, Easton KL, et al. Assessment and management of asthma and chronic obstructive pulmonary disease in Australian general practice. Aust Fam Physician. 2017;46(6):413–419.
- Simeone JC, Luthra R, Kaila S, et al. Initiation of triple therapy maintenance treatment among patients with COPD in the US. Int J Chron Obstruct Pulmon Dis. 2017;12:73–83.
- Sundh J, Aberg J, Hasselgren M, et al. Factors influencing pharmacological treatment in COPD: a comparison of 2005 and 2014. Eur Clin Respir J. 2017;4(1):1409060.
- Cazzola M, Rogliani P, Calzetta L, et al. Triple therapy versus single and dual long-acting bronchodilator therapy in chronic obstructive pulmonary disease: a systematic review and meta-analysis. Eur Respir J. 2018;52(6):1801586.
- Kwak MS, Kim E, Jang EJ, et al. The efficacy and safety of triple inhaled treatment in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis using Bayesian methods. Int J COPD. 2015;10(1):2365–2376.
- Langham S, Lewis J, Pooley N, et al. Single-inhaler triple therapy in patients with chronic obstructive pulmonary disease: a systematic review. Respir Res. 2019;20(1):242.
- Zheng Y, Zhu J, Liu Y, et al. Triple therapy in the management of chronic obstructive pulmonary disease: systematic review and meta-analysis. BMJ. 2018;363:k4388.
- WHO Collaborating Centre for Drug Statistics Methodology. List of DDDs combined products [Internet]. Available from: https://www.whocc.no/ddd/list_of_ddds_combined_products/)
- Izquierdo JL, Cosio BG. The dose of inhaled corticosteroids in patients with COPD: when less is better. Int J Chron Obstruct Pulmon Dis. 2018;13:3539–3547.
- Tervonen T, Hawken N, Hanania NA, et al. Maintenance inhaler therapy preferences of patients with asthma or chronic obstructive pulmonary disease: a discrete choice experiment. Thorax. 2020;75(9):735–743.
- PBAC. 5.03 FLUTICASONE FUROATE 100 mcg + UMECLIDINIUM 62.5 mcg + VILANTEROL 25 mcg, powder for inhalation, 30 actuations, Trelegy® Ellipta®, GlaxoSmithKline Australia. 2017. https://www.iqvia.com/.
