ABSTRACT
Background
Quantification of COVID-19 burden may be useful to support the future allocation of resources.
Objective
To evaluate the public health impact of COVID-19 in French ambulatory patients with at least one risk factor for severe disease.
Study design
A Markov model was used to estimate life years, costs, number of hospitalisations, number of deaths and long/prolonged COVID forms over a time horizon of 2 years. The hospitalisation probabilities were derived from an early access cohort, and the hospitalisation stay characteristics were derived from the French national hospital discharge database. Several scenario analyses were conducted.
Results
The number of hospitalisations reached 256 per 1,000 patients over the acute phase (first month of simulation), and 382 per 1,000 patients over 2 years. The number of deaths was 37 per 1,000 patients, and the number of long/prolonged COVID forms reached 407 per 1,000 patients. These translated into a reduction of 0.7 days of life per patient in the first month, with an associated cost of €1,578, and a reduction of 27 days of life over the time horizon, with an associated cost of €4,280. The highest burden was observed for patients over 80 years old, and those not vaccinated. The scenarios with a less severe situation or new treatments available showed a non-negligible burden reduction.
Conclusion
This study allowed us to quantify the considerable burden related to COVID-19 in infected patients, with at least one risk factor for severe form. Strategies with the ability to substantially reduce this burden in France are urgently required.
Introduction
The pandemic of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), as declared a pandemic by WHO in March 2020, has resulted in approximately 410 million confirmed cases and 5.8 million deaths globally until mid-February 2022[Citation1].
Much like the rest of Europe, France has been hit hard by the pandemic and went into lockdown on 17 March 2020, until 11 May 2020, at the end of the first wave. Since then, several additional waves have hit France, and by mid-February 2022, more than 21.8 million infections have been confirmed, with more than 135000 deaths[Citation1]. Unprecedented responses have been put in place, such as national lockdown resulting in a wide stay-at-home policy, curfews, closure of the non-essential shops, closure of the borders and the application of other measures aiming to reduce the spreading of the disease.
‘Several measures have been in force since 26 November 2021 to slow down the new wave of the epidemic, including tightened protective measures’. Some of those measures are compulsory: mask wearing in all indoor public spaces, including those where the COVID-19 certificate is required and in busy or crowded outdoor spaces while other measures are recommended: hand washing, regular airing, coughing and sneezing into elbow and using single-use tissues[Citation2].
In France, the first COVID-19 vaccine injection took place in December 2020, and by mid-February 2022, five vaccines are available[Citation3] (mRNA-1273/Spikevax® commercialised by Moderna, BNT162b2/Comirnaty® by Pfizer/BioNTech, Ad26.COV2.S/ by Janssen, Nuvaxovid by Novavax and AZD1222 by Oxford/AstraZeneca). In December 2021, the vaccination was open to children 5–11 years old. By mid-February 2022, more than 53 million persons, i.e., almost 93% of the adult population, have received the two first doses[Citation4]. A booster has recently been proposed to reinforce vaccine protection.
In addition to prevention, several treatments have received a marketing authorisation or were available through a temporary use authorisation in France. These included four monoclonal antibody treatments (casirivimab & imdevimab by Roche, bamlanivimab & etesevimab by Lilly, tixagevimab & cilgavimab by AstraZeneca, and sotrovimab by GSK), two antivirals (remdesivir by Gilead and PF-07321332/ritonavir by Pfizer), one anti-inflammatory (anakinra by Sobi), one interleukin-6 neutralising antibody (tocilizumab by Roche), and dexamethasone.
Most of these treatments are not available for the general population, as their indication is restricted to hospitalised patients with severe forms of the disease or to ambulatory mild or moderate COVID-19 in patients at risk to develop a severe form. Risk factors for a severe form are numerous, and these patients may include older patients, patients with obesity, cardiovascular disease, hypertension, chronic lung disease including asthma, type 1 or 2 diabetes mellitus, chronic kidney disease, or immunosuppressed patients. Also, because of a lack of neutralizing effect on the increasingly dominant Omicron variant, national recommendations recently changed, with no more use of bamlanivimab and etesevimab combination, restriction of use of casirivimab & imdevimab combination to patients excluding variants where it lost efficacy, e.g., Omicron, and use of sotrovimab in situations due to Omicron variant or to unknown variant.
Globally, the sanitary situation is highly dynamic, with new variants, increased protection of severe forms by vaccines and changes on treatment positioning. Therefore, calculations of the disease burden of COVID-19, in terms of hospitalisations, death and costs, may be useful in several dimensions: supporting the future allocation of resources, comparing COVID-19 with other diseases in the population and across populations.
We aimed to provide the first elements of the answers by presenting the results of a simple but flexible model to evaluate the public health impact of COVID-19 in French ambulatory patients with at least one risk factor for severe disease.
Methods
Model structure
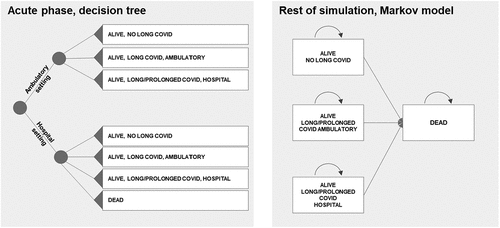
To ensure an appropriate simulation of patient pathways, the model contains two parts: the first one models the acute phase of the COVID-19 infection through a decision tree and the second one models the remaining simulation duration of the patient through a Markov model (see ).
This approach is in line with the one used by the Institute for Clinical and Economic Review’s (ICER) for the remdesivir model, focusing on hospitalised patients [Citation5,Citation6]; it allows the use of treatment and its associated outcomes over a short-term period, 1 month in this case, while still allowing the model to consider the long-term consequences of COVID-19 on healthcare costs. The decision tree consists of two treatment settings (outpatient and hospital settings) and four outcomes. The Markov model consists of four health states that mirror the four outcomes that patients can achieve in the decision tree.
Patients enter the model with a COVID-19 infection and at least one risk factor for severe disease. In the first month, they may be hospitalised or remain in outpatient settings. The hospital setting distinguishes patients who require or not limited to low-flow oxygen, those with non-invasive ventilation and those with mechanical ventilation; each status has an impact on hospital costs and mortality. At the end of this acute phase, the patient can recover without any health issues (‘alive, no long COVID’), with moderate long-term health issues (‘alive, long COVID, ambulatory’), severe health issues (‘alive, long/prolonged COVID, hospital’), or die. Of note, it is assumed that patients cannot die in the outpatient health state as evidence shows that most patients die during a hospital stay.
Patients who leave the acute phase alive enter the Markov model in the corresponding health state. While a clear definition of long COVID does not exist yet, it appears to refer to long-term symptoms and/or organ damage experienced by patients, post-infection [Citation7–9]. This notion of long-term symptoms is here combined with prolonged COVID in this model, which consists of patients for whom the COVID hospitalisation lasted more than 1 month.
Death is an absorbing health state in which patients remain until the end of the model’s time horizon.
Time horizon, cycle length and discounting
It is assumed that the acute phase has a duration of 1 month (30.44 days). As the effects of the treatment and long-term consequences go beyond this phase, the model assumes that costs may be cumulated until the end of year two.
The Markov model considers a cycle length of 1 year. Given the lack of evidence on long-term outcomes, a more granular cycle is not necessary. The model applies half-cycle corrections, resulting in transitions occurring in the middle of a cycle rather than at the end of a cycle.
The model discounts costs and benefits across time at 2,5%, in alignment with the French health authorities’ guidelines [Citation10].
Population characteristics at model entry
This model attempts to simulate the trajectory of patients who, at model entry, are infected, at risk of a severe form of COVID-19, and not treated.
Evidence suggests that patients’ age may affect the risk of hospitalisation and the risk of death while one is in hospital [Citation11,Citation12] and is the main factor in the development of a severe form [Citation13]. Therefore, the model is stratified by age, considering the following subgroups: less than 50 years old, between 51 and 60 years old, between 61 and 80 years old, and over 80 years old. The baseline distribution of patients in age groups, as well as the gender, is derived from an early access cohort of patients treated with Ronapreve® (casirivimab & imdevimab), to be as close as possible to real life [Citation14]. This cohort was put in place in March 2021 in France, for infected patients at risk for a severe COVID-19 form to benefit from a single dose of treatment before the EU marketing authorisation was given (TUA, Temporary Use Authorisation cohort). The follow-up of this cohort resulted in the collection of precious data on patients’ characteristics as well as hospitalisation rates, by age group. By mid-2 December 2021, 118 exposed patients were included in the cohort. The mean age was 62,7 (median 65) years, ranging from 12 to 104 years, as shown in .
Table 1. Model inputs
Event and transition probabilities
Acute phase – hospitalisation rate
Age-specific hospitalisation rates for the first month were derived from the same cohort of patients, to inform the probability of hospitalisation for outpatients treated with Ronapreve® monoclonal antibody. The results show that, on average, 7.93% of patients who received Ronapreve® were hospitalised at least 1 day during the first month, ranging from 3.43% for the youngest group, to 15.25% for the oldest group. In the Ronapreve® clinical trial [Citation15], the proportion of patients with one or more than one hospitalisation or all-cause death through day 29 was 1.0% in the active group (Ronapreve® 1200 mg) vs 3.2% in the placebo group, resulting in a risk ratio of 0.31. This ratio was applied to the hospitalisation rate found in patients treated with Ronapreve® in real life; the estimated hospitalisation rate was 25.59% for patients with no treatment, ranging from 11.23% to 49.20% depending on the age group.
Acute phase – oxygen support in hospital
The model sources age-specific information on the proportion of patients who require standard or no oxygen support, non-invasive ventilation, or mechanical ventilation from the French National Hospital Discharge Database (PMSI; Programme de Médicalisation des Systèmes d’Information), considering a National Health Insurance (NHI) perspective. The PMSI hospital discharge database covers all hospitalisations in the public and private sectors involving short-term stays in medical, surgical or obstetric facilities, representing more than 95% of all hospitalisations in France [Citation16]. Patients were tracked across multiple hospitalisations through a unique anonymous patient identifier, which is retained until the patient dies. The analysis included all patients with a documented ICD-10 code for any form of COVID-19, with at least one risk factor, and all hospital admissions from the PMSI MCO 2020 database with a principal or associated diagnosis of COVID-19 were extracted. More details on the methodology of this analysis are presented in the Supplemental Appendix.
Based on the results of this analysis, the model considers that 89.5% of the patients hospitalised receive standard oxygen or no oxygen support, while 2.1% receive non-invasive ventilation, and 8.4% receive mechanical ventilation.
Acute phase – distribution of health states
The age-specific distributions of health states in which patients leave this acute phase, hospitalised or not during the acute phase, were derived from the PMSI study or based on expert opinion.
Similar to the calculation of the length of hospitalisation, the model allows the probability of hospital death to be conditional to oxygen support.
Rest of life – Markov model
The model uses French life table statistics [Citation17] to inform the probability of natural death by age and gender, for patients in the Markov model. Although patients who become infected may be a subpopulation of the population with a greater number of comorbidities or may leave the acute phase with health issues, no additional decrease in survival was accounted for in the Markov model, conservatively.
Costs
Acute phase
The model does not consider any cost for patients treated in outpatient settings, but hospitalised patients incur hospital costs, stratified by the level of oxygen support and age group. These are sourced from the PMSI study, considering all hospital stays that lasted less than 30 days.
Rest of life – Markov model
No costs are accounted for healthcare in patients who are alive with no long COVID after the acute phase. Patients with long or prolonged COVID will require additional healthcare resources; the model conservatively assumes a duration of 2 years for these costs, in the absence of any longer-term information.
The cost of long COVID treatment in outpatient settings was based on literature review and clinical expert opinion [Citation18]. It was assumed that patients could experience several types of symptoms (pulmonary, cardiologic, neurologic, mental health, digestive, dermatologic, ear nose and throat disorder symptoms, and other symptoms), leading to specialist referrals, investigations, and delay in return to work in a certain proportion as described in Supplemental Appendix.
The cost for long/prolonged COVID treated in hospital was based on the PMSI study (Supplemental Appendix).
Model outcomes
The outcomes calculated by the model include life years, costs, number of hospitalisations, number of deaths and long/prolonged COVID forms.
Model scenarios
In addition to the base case analysis, several scenarios were run, to explore the impact of the model results.
Age is a major determinant of the burden, as observed in the hospitalisation rates, the death rates, or the associated costs. Therefore, it was important to present the scenario ‘AGE’ with results for each age group separately.
The scenario ‘CARE IMPROVEMENT’ considers the improvement of care between 2020 and 2022. Indeed, the hospital mortality rate is based on data from 2020, when the clinicians first met this virus and when hospitals were not fully equipped or organised to best treat their patients, and where no therapies had yet proven to improve outcome; it is likely that the substantial changes in supportive care and management, and the use, or the arrival of treatments have led to a reduction of hospital mortality. Therefore, this scenario considers a 20% decrease in-hospital mortality.
It was also considered important to explore the impact of treatment of mild-to-moderate COVID-19 in ambulatory patients with at least one risk factor. The model allows to consider hospitalisation reductions as well as long/prolonged COVID occurrence reduction by applying a risk ratio (RR) and to consider administration costs for any treatment. Therefore, in the scenario ‘TREATMENTS’, several RR were tested, including RR = 0.1, RR = 0.3 and RR = 0.5, assuming that some patients would receive the treatment in the ‘hospital at home’ settings, while others go to hospital as part of day care treatments without incurring a hospitalisation.
The hospitalisation rates were based on a cohort of patients mostly infected with the delta variant (the year 2021), and the inputs from the claims database analysis are mainly derived from data of patients infected with the alpha variant (the year 2020). Therefore, it was considered important to explore the impact of a less severe variant, by attempting to replicate the reduction in the severity of the Omicron variant as an example. Therefore, a scenario ‘OMICRON’ was run, with a 50% reduction in hospitalisation rates and associated mortality rates, as well as a 50% reduction of long/prolonged COVID after a hospitalisation, and a reduction of 100% of long/prolonged COVID after an infection treated in ambulatory settings.
Although the proportion of vaccinated patients in the TUA cohort is not known, it is very likely that the majority had received a complete immunisation schedule. Indeed, this cohort was put in place for patients at risk of a severe form of COVID-19, i.e., immunocompromised patients, those with a chronic debilitating condition, or aged more than 80 years. Therefore, the scenario ‘NON-VACCINATED’ attempts to evaluate the burden of the non-vaccinated patients, by increasing the hospitalisation rates and the associated mortality rates by 50%. This could also apply to the unresponsive fully vaccinated patients, i.e., those severely immunocompromised.
A last scenario considers that the model entry population is the general population, not limited to patients at risk of severe form. In the scenario ‘GENERAL POPULATION’, all inputs based on PSMI analysis are updated with results considering all hospital stays in France (not only those of patients at risk) and a reduction of 60% of the hospitalisation rates and the associated mortality rates.
Results
All base case results are presented in .
Table 2. Model results for the base case and scenarios
Over the model time horizon, the number of hospitalisations reached 256 per 1,000 patients in the acute phase and 382 per 1,000 patients overall. The number of deaths in the acute phase was 37 per 1,000 patients, and the number of long/prolonged COVID forms reached 407 per 1,000 patients. These translated into a reduction of 0.7 days of life per patient in the acute phase, with an average cost of €1,578, and a reduction of 27 days of life over the time horizon, with an average cost of €4,280.
The scenario ‘AGE’ illustrates how group age impacts these results. The hospitalisation rate in the youngest group (<50 years) was 112 per 1,000 patients and increased to 492 per 1,000 patients for the oldest group (>80 years). A similar tendency, yet much more pronounced, was observed for mortality rates, with numbers below 10 per 1,000 patients for the youngest groups, increasing to more than 150 per 1,000 patients for the oldest groups, reflecting the higher hospitalisation and mortality rates for oldest patients. These results are reflected in the estimated average acute costs over the first month of simulation, ranging from €705 to €2,507. In contrast, the impact of age on the number of long/prolonged COVID forms was limited. The impact of age on costs was important in the acute phase, but the oldest group incurred fewer costs when the full-time horizon was considered. This is explained by three reasons. First, patients above 80 years old have a much higher death rate and die earlier than younger patients in the model, providing less opportunity to accumulate costs. Second, their cost per hospital stay in the acute phase is smaller, and their probability to experience a long/prolonged COVID form is smaller, when compared to younger patients. The results on life duration show how COVID-19 impact is large in the oldest group, with almost 3 months and a half of life lost due to COVID-19 including almost 3 days over the first month, and on the 61–80 group, with more than 1 month of life lost due to COVID-19 including almost 1 day over the first month.
The scenario ‘CARE IMPROVEMENT’ results in a reduction of the number of deaths per 1,000 patients from 37 to 29, and a similar number of hospitalisations, long/prolonged COVID forms, and costs. Of note, the number of total hospitalisations per 1,000 patients is higher by 1 (383 vs. 382 in the base case), as more patients alive also means more long-term complications and/or sequelae. The reduction in life duration lost is 0.1 days in the acute phase, accumulating to 21.7 days over the full-time horizon, leading to an additional 1 week of life gained, thanks to the care improvement.
The scenarios ‘TREATMENTS’ show the potential benefits of treatments arriving on the market at risk population with mild or moderate COVID-19. As expected, these benefits increase when RR is smaller, i.e., when the treatment effect is higher. An RR of 0.5 automatically reduces the number of hospitalisations by 50%, while an RR of 0.1 reduces this number by 90%. As no treatment effect is considered on mortality, the reduction in the number of deaths is similar to the reduction in hospitalisations. The impact on costs is similar to the impact on clinical outcomes and so is the impact on life duration lost due to COVID-19.
The scenario ‘OMICRON’, based on a 50% reduction in the hospitalisation and mortality rate in the acute phase, leads to a reduction in the number of deaths per 1,000 patients by more than 75%; the impact of long/prolonged COVID forms is even more pronounced, with a more than 90% reduction. The life duration lost due to COVID-19 is also reduced to 0.2 days in the first month and to 6.8 days over the time horizon. This translates into cost important reductions.
The scenario ‘NON VACCINATED’ illustrates an opposite case, with an increase in the number of hospitalisation per 1,000 patients by 50% in the acute phase and by 34% in total, with a number of deaths multiplied by 2.24. The impact on number of long/prolonged COVID forms is limited, because of diminution of survivors. The life duration lost due to COVID-19 is increased, to 1.6 days in the first months and to 2 months over the time horizon. This translates into cost important increases.
The scenario ‘GENERAL POPULATION’ results in a reduction of the clinical and economic burden, with fewer hospitalisations and deaths per 1,000 patients, and fewer costs. Here again, the impact on the number of long/prolonged COVID forms is limited. The costs are reduced to €470 per patient in the acute phase, and €2,463 in over the full-time horizon, and the life duration lost due to COVID-19 to 0.1 days in the acute phase and 3.6 days over the full-time horizon.
Discussion
Assessing the public health impact of COVID-19 in French hospitalised patients with at least one risk factor for severe disease is important to help health policymakers in evaluating and making decisions about preventive programmes, especially in the context of this pandemic, with limited healthcare resources.
One of the main inputs of this model is the hospitalisation rate, which is derived from an existing cohort of patients at risk for a severe form. First, it is worth mentioning that those hospitalisation rates were estimated at a certain point in time of the pandemic. Hence, they are related to a specific situation in time where several parameters such as the vaccination status, the proportion of variants circulating, and the health policies were not completely comparable to what they are today. This may have a considerable impact on hospitalisation rates. Also, this cohort allows the results to reflect as closely as possible real-life rates, although limitations should be acknowledged. It is possible that the proportion of immunocompromised patients is higher than in real life, as the clinicians may have prioritized these patients at the beginning of the recruitment, which was mainly done through hospital networking. This could lead to a possible overestimation of the hospitalization rate. On the other hand, the hospitalisation status was not known for a non-negligible proportion of patients. Therefore, to compensate for missing data, it was considered appropriate to assume these patients were not hospitalised.
Despite the dynamic health context, this public health impact model allows us to inform on this burden. The results suggest that this burden is considerable, with more than 380 hospitalisations and more than 400 long/prolonged COVID forms per 1,000 patients after the infection. This translates into a reduction of life years by almost 1 month over the model time horizon (2 years and 1 month) on average, and costs exceeding €1,500 per patient in the acute phase, and €4,200 per patient over the full-time horizon.
This burden is considerable for this at-risk population, especially for the oldest patients, for whom the reduction of life duration reaches almost 3 days of life in the first month, as per the high hospitalisation and death rates in this population. Indeed, almost 500 hospitalisations are simulated in the first month per 1,000 patients infected. Moreover, the failure to account for deaths in residential care facilities for the elderly (French EHPAD) may underestimate this burden. The total reduction of life duration is even more dramatic, as the model shows that these infected patients over 80 years old would live 3 months less than patients without COVID-19. Also, as no increase in the death rate was accounted for patients suffering from long-term health issues, this reduction might be underestimated.
In 2 years, improvements have been observed in hospitals, in terms of management, care, availability of treatments, etc. Although the PMSI data are not yet available, it was relevant to consider that these actions resulted in a decrease in the mortality rate. And as shown in the corresponding scenario, the impact is important in terms of survival, and reduction of life duration lost due to COVID-19. However, further research is needed to assess the impact on survival in critical care patients with COVID-19. For example, it is imperative to investigate how requirement for intensive care units may negatively affect the outcomes and to identify strategies to prevent healthcare system to be overloaded in the following waves.
Another way to improve survival in this at-risk population is to introduce early treatments in ambulatory mild and moderate COVID-19. The model allows the presentation of several scenarios in which a treatment is used in these patients. Although these scenarios do not consider any adverse event of the treatments, considered to be negligible for most of them, the results provide a good opportunity for decision makers to appreciate the impact of a treatment reducing the number of hospitalisations on the overall burden. The results showed treatments would significantly reduce the number of hospitalisations and survival, as well as healthcare costs during the acute phase. Of note, inputs related to clinical outcomes after hospitalisation (oxygen support type in hospital) may depend on the presence of treatment or not. However, the model assumes that the values of clinical inputs after hospitalisation are not conditional on treatment presence, due to a lack of precise evidence to inform the matter. As it is likely that such treatments are associated with benefits in terms of the severity of the disease, reflected in less and shorter hospitalisations with less invasive treatments, this assumption is likely to be conservative. Importantly, it is not clear whether the efficacy of these treatments will be as high on reduction on hospitalisation with newly identified variants.
The model does not distinguish any input between variants of the disease, while they differ in terms of transmissibility, aggressivity, vaccine escape, ability to evade diagnostic detection or ability to cause more severe disease. However, it should be reminded that the model considers two main sources for inputs. On one hand, all inputs derived from the PMSI study were based on the year 2020, where the variant Alpha was predominant. On the other hand, the aged-based hospitalisation rates are sourced from a cohort of patients treated with Ronapreve® between March and December 2021; these rates were therefore mainly driven by the Delta variant. Therefore, it is expected that the base case results presented in this analysis are reflecting a situation with the main variants that France faced in 2020 and 2021. The scenario ‘OMICRON’ attempts to evaluate the impact of a variant causing less severe disease than these previous strains, as it was shown to be the case for the Omicron variant, even in those who are unvaccinated or who have not had a prior COVID-19 infection [Citation19]. The results show how important the impact might be on the disease burden, assuming the considered assumptions are realistic.
Also, it should be noted that the model does not distinguish any input between the vaccination status of the patients at model entry. While it is clear that most inputs derived from the PMSI study reflect a population without any vaccination, this is not known for the hospitalisation rates. Indeed, the vaccination status was not collected during the Ronapreve® TUA cohort. However, as these patients were at risk for severe form, it could be assumed that most of them had received at least two doses of vaccine. A scenario was run to evaluate the impact of the disease on a population with more risks of hospitalisation and mortality at the hospital. As expected, the burden is even more considerable especially when it comes to survival.
Also, the model is stratified by age but is not stratified by comorbidity or immunodepression status, although these are well-identified severity risk factors. Authors from the Mayo Clinic developed the monoclonal antibody screening score, a weighted score reflecting the relative risk by comorbidity and by cumulative comorbidities, to stratify patients’ risk profiles and identify eligible patients who can best benefit from early outpatients treatment [Citation20,Citation21]. Nevertheless, it was considered that this would add a lot of complexity to the model, with too many assumptions required, and too various at risk conditions, in contrast to the limited benefits in terms of the precision of the results. It was then considered not reasonable to introduce more uncertainty in the model, especially since the age group is the most important risk factor.
Of note, the model structure assumes that patients cannot contract COVID-19 again, while recurrent COVID-19 infections occur through the reduction of immunity over time, lack of immune response or the emergence of new variants. However, a proportion of the costs of reinfections is reflected in the costing of the long/prolonged COVID. Indeed, some stays related to a second (or more) infection may be considered as stays due to long COVID, in the PMSI database. On one hand, this may slightly overestimate the cost of long/prolonged COVID. But on the other hand, the cost of long/prolonged COVID is assumed to last no more than 2 years in the model.
In addition, the model does not allow patients to transition across health states in the Markov model due to a lack of evidence on the issue. Moreover, the model separates patients with long COVID after the acute phase into two health states, based on the setting (outpatient or hospital). The continuum of severity and duration of long COVID may suggest the presence of additional health states. These simplifications may lead to incorrect calculation of healthcare costs for patients who leave the acute phase with a long COVID. However, it is unclear how this would affect the results of the model.
Finally, the last presented scenario illustrates the burden of COVID-19 in infected patients, but independently of their risk factors. As expected, the burden per patient is reduced, but it is unclear how this would affect the total burden for the entire French population, in a context where the number of persons infected, and the proportion of those at risk within this population, are not well known. However, these are important results for health policymakers in evaluating and making decisions about preventive and treatment programmes, and in view of the ecosystem and the current health context, it seems appropriate to shed light on and gain a better understanding of the resources committed during this pandemic.
In conclusion, this study details the considerable burden related to COVID-19 in infected patients, with at least one risk factor for severe form of the disease. Strategies with the ability to substantially reduce this burden in France are urgently required, especially in the elderly.
Supplemental Material
Download MS Word (115.1 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/20016689.2022.2082646
Additional information
Funding
References
- COVID-19 data repository by the center for systems science and engineering (CSSE) at Johns Hopkins University. [cited 2022 Feb 15] Available from: https://github.com/CSSEGISandData/COVID-19
- Coronavirus - Protective measures. [cited 2022 Feb 15] Available from: https://www.diplomatie.gouv.fr/en/coming-to-france/coming-to-france-your-covid-19-questions-answered/coronavirus-protective-measures/
- Covid-19 Vaccine Tracker. cited 2022 Feb 15 Available from: https://covid19.trackvaccines.org/country/france/
- Vaccines. [cited 2022 Feb 15] Available from: https://www.gouvernement.fr/info-coronavirus/vaccins
- COVID-19. An assessment of COVID-19 treatments, including remdesivir. [cited 2022 Feb 15] Available from: https://icer.org/explore-our-research/policy-papers/covid-19/#timeline
- ICER Releases Draft Evidence Report on Treatments for COVID-19 [cited 2022 Mar 4] Available from: https://icer.org/news-insights/press-releases/icer-releases-draft-evidence-report-on-treatments-for-covid-19/
- Vittori A, Lerman J, Cascella M, et al. COVID-19 pandemic acute respiratory distress syndrome survivors: pain after the storm? Anesth Analg. 2020;131(1):1–10.
- Wu Y, Xu X, Chen Z, et al. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav Immun. 2020;87:18–22.
- Dernières informations sur les effets cliniques à long terme de la COVID-19. [cited 2022 Feb 15] Available from: https://www.who.int/docs/default-source/coronaviruse/risk-comms-updates/update-54-clinical-long-term-effects-of-covid-19-fr.pdf?sfvrsn=3e63eee5_14
- HAS. Choix méthodologique pour l’évaluation économique à la HAS. 2020. Accessed 15 February 2022. Available from: https://www.has-sante.fr/jcms/r_1499251/fr/choix-methodologiques-pour-l-evaluation-economique-a-la-has
- Docherty AB, Harrison EM, Green CA, et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO clinical characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985.
- Madjid M, Safavi-Naeini P, Solomon SD, et al. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020;5(7):831–840.
- Semenzato L, Botton J, Drouin J, et al. Chronic diseases, health conditions and risk of COVID-19-related hospitalization and in-hospital mortality during the first wave of the epidemic in France: a cohort study of 66 million people. Lancet Reg Health Eur. 2021;8:100158.
- Autorisation temporaire d’utilisation de cohorte. Résumé du rapport de synthèse périodique n°5. Accessed 15 February 2022. https://ansm.sante.fr/uploads/2022/01/21/20220121-atuc-ronapreve-resume-rapport-n05.pdf
- Weinreich DM, Sivapalasingam S, Norton T. REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19. N Engl J Med. 2021 Jan 21;384(3):238–251.
- Bezin J, Duong M, Lassalle R, et al. The national healthcare system claims databases in France, SNIIRAM and EGB: powerful tools for pharmacoepidemiology. Pharmacoepidemiol Drug Saf. 2017;26:954–962.
- INSEE. Table de mortalité par sexe, âge et niveau de vie. Disponible en ligne. Accessed 15 February 2022. https://www.insee.fr/fr/statistiques/3311422?sommaire=3311425, [cited 2022 Feb 15]
- Heightman M, Prashar J, Hillman TE, et al. Post-COVID-19 assessment in a specialist clinical service: a 12-month, single-centre, prospective study in 1325 individuals. BMJ Open Respir Res. 2021 Nov;8(1):e001041.
- Davies MA, Kassanjee R, Rosseau P, et al. Outcomes of laboratory-confirmed SARS-CoV-2 infection in the Omicron-driven fourth wave compared with previous waves in the Western cape Province, South Africa. Tropical Medicine & International Health . 2022 Dec 1;12:22269148. Preprint
- Bierle DM, Buckmeier J, Kudrna C, et al. Monoclonal antibody selection score for allocation of anti-spike monoclonal antibodies to high risk patients with COVID-19. Pers Commun. 2021. 12 1–8 .
- Bierle DM, Ganesh R, Wilker CG, et al. Influence of social and cultural factors on the decision to consent for monoclonal antibody treatment among high-risk patients with mild-moderate COVID-19. J Prim Care Community Health. 2021;12:21501327211019282.