ABSTRACT
Background
Clinician’s choice of hypoallergenic formulas in the first-line management of cow’s milk protein allergy (CMPA) should be informed by evidence on clinical efficacy and cost-effectiveness.
Objective
We compare the cost-effectiveness of amino acid-based formula (AAF), extensively hydrolyzed casein formula with Lactobacillus rhamnosus Gorbach Goldin (EHCF+LGG), extensively hydrolyzed whey formula (EHWF), and rice hydrolyzed formula (RHF) in non-breastfed children in France.
Methods
Immunotolerance and atopic manifestations’ prevalence were based on a prospective non-randomized study with a 36-month follow-up. Resource utilization was sourced from a survey of French clinicians, and unit costs were based on national data. Costs and health consequences were discounted at 2.5% annually. Results were reported using the Collective and French National Health Insurance perspectives.
Results
Children receiving EHCF+LGG were predicted to require less healthcare resources, given their reduced prevalence of CMPA symptoms at 3 years. In the base case, EHCF+LGG led to savings of at least €674 per child compared to AAF, EHWF, and RHF at 3 years, from both perspectives. Nutrition had the highest economic burden in CMPA, driven by hypoallergenic formulas and dietetic replacements costs. Results were robust to one-way and probabilistic sensitivity analyses.
Conclusions
EHCF+LGG was associated with more symptom-free time, higher immune tolerance, and lower costs.
Introduction
The benefits of maternal milk have been widely recognized by the scientific community [Citation1,Citation2] but there are situations in which breastmilk is not sufficient or parents choose not to breastfeed [Citation3]. Therefore, formulas containing cow’s milk proteins (CMP) are given to infants and cow’s milk protein allergy (CMPA) can develop in few of them. When CMPA occurs, hypoallergenic formulas replace standard formulas. CMPA is one of the most common food allergies worldwide in the first year of life [Citation4,Citation5]. Its prevalence is thought to range from 0.5 to 3% in infants born in developed countries, although there is substantial variability depending on diagnostic criteria [Citation6,Citation7]. In studies using self-reported criteria, prevalence ranges from 1.2 to 17% [Citation8] and there is some evidence suggesting that the condition is becoming more frequent in adulthood [Citation6]. CMPA manifests in a range of gastrointestinal, dermatological, and respiratory symptoms that can be detrimental to children’s nutritional status and development, leading to unforeseen health costs [Citation6,Citation9]. CMPA is regularly categorized into immunoglobulin E (IgE)-mediated and non-IgE-mediated symptoms. IgE-mediated reactions are characterized by the occurrence of atopic manifestations (AM) within 1 to 2 hours after the allergen’ ingestion. Non-IgE-mediated symptoms present within hours to days [Citation10]. European directives recommend the use of an extensively hydrolyzed formula as the first-line management of CMPA in non-breastfed children except in infants with anaphylactic reactions or severe enteropathy suggested by faltering growth for whom an amino acid-based formula (AAF) is primarily recommended [Citation9,Citation11]. Because several formulations are available, clinician prescription should be informed by efficacy and cost-effectiveness data, ensuring the best use of resources [Citation12]. One prospective non-randomized study found extensively hydrolyzed casein formula (EHCF) with or without added Lactobacillus rhamnosus Gorbach Goldin (LGG), now renamed Lacticaseibacillus rhamnosus, increased the likelihood of cow’s milk tolerance compared with AAF, rice hydrolyzed formula (RHF), and soy formula at the 12-month follow-up [Citation13]. At a later stage, a randomized control trial recruiting children with IgE-mediated CMPA found that EHCF+LGG was associated with a 23% (95% CI, 10 to 36%) reduction in the incidence of AM and a 20% higher probability of becoming cow’s milk tolerant (95%CI, 22 to 43%) at 36 months follow-up, compared to EHCF alone [Citation14]. A recent prospective cohort study compared the incidence of AM and cow’s milk tolerance over 36 months, in children using different formulas. At this timepoint, children receiving EHCF+LGG were statistically significantly less likely to have any AM and had a higher probability of being tolerant to cow’s milk [Citation15].
Health technology assessment has been widely used in France as a decision-making tool informing the commissioning of medical technologies. Assessments have been mostly focused on innovative drugs and medical devices. Since its creation in 2005 [Citation16] the Haute Autorité de Santé (HAS) has greatly contributed to developing methodological guidance in the use of economic evaluation [Citation17] and promoting transparency in evidence-based healthcare decision-making [Citation12]. However, non-prescription healthcare products are commonly out of scope for HAS decision-making as they are generally not reimbursed, representing a burden for patients and families. This emphasizes the importance of including the Collective perspective in cost-effectiveness analyses of these products, depicting the consequences of efficient clinical prescriptions along with families’ out of pocket expenses.
When CMPA is developed, hypoallergenic formulas replace standard infant formulas and it is essential that clinical prescription of these formulas is enlightened by rigorous criteria of both efficacy and cost-effectiveness. Two economic evaluations of whey-based hydrolyzed formula have been published before in the French setting, concerning its impact on atopic dermatitis prevention [Citation18,Citation19]. However, these studies became outdated as new hypoallergenic formulas were introduced in the market and not all comparators are considered in these studies.
Previous health economic analyses have explored the head-to-head cost-effectiveness comparison of hypoallergenic formulas in acquiring tolerance to CMP in Italy [Citation20], Spain [Citation21], Poland [Citation22] and the UK (UK) [Citation23] but no such study exists in France. The objective of this economic evaluation is to assess the cost-effectiveness of commonly used hypoallergenic formulas (AAF, EHCF+LGG, extensively hydrolyzed whey formula [EHWF], and RHF) in infants and young children presenting with IgE-mediated CMPA in France, applying the most recent evidence in the field, at time of publication. The Consolidated Health Economic Evaluation Reporting Standards (CHEERS) framework for reporting economic evaluations was used to prepare this report [Citation24].
Materials and Methods
Model structure
We developed a trial-based decision analytic cohort model in Microsoft Excel to simulate the use of hypoallergenic formulas to manage IgE-mediated CMPA in non-breastfed children in France. The model structure was based on a published cost-effectiveness analysis in the UK [Citation23]. The analysis uses the annual probabilities of AM and acquisition of tolerance to CMP from a prospective non-randomized trial, over 3 years. The trial informing the model compares the use of AAF, EHCF+LGG, EHWF, and RHF [Citation15]. Infants and children usually present CMPA and potentially acquire tolerance over a 3-year period, which aligns with the duration of follow-up used by a recent publication [Citation25]. Soy formulas are no longer available in France and were not included in the analysis.
The model simulates a cohort of 5-month-old community-based infants with IgE-mediated symptoms of CMPA, who may develop other AM (eczema, asthma, rhinoconjunctivitis, or allergic urticaria) or may become symptom free [Citation15]. Health states were modelled as mutually exclusive and exhaustive, with annual probabilities of belonging to each health states adding to 1. CMPA management costs such as those for health care and dietetic replacements were attributed to infants in each health state and accumulated over time. Because we had no information of the number of children having multiple symptoms, we specifically modelled children according to their main AM, as defined in the clinical trial. Similarly, no information was provided about the proportion of infants presenting with AM after symptom-free periods so this was not modelled directly. Those becoming immunotolerant were assumed not to present AM and were discontinued from hypoallergenic formula. Mortality due to CMPA or hypoallergenic formula intake (unlikely event) was not considered in the analysis, as any difference between cohorts would be negligible. The model structure is represented in .
Figure 1. Model structure22.
Health resources used were obtained from French pediatricians experienced in the management of CMPA and AM, using a purpose-built questionnaire. The analysis considered two perspectives of costs:
1) Collective: The French National Authority for Health (HAS) refers to the ‘Collective’ perspective [Citation12] as that including “all the individuals or institutions affected in the production of an intervention. In the present analysis, this perspective includes overall costs incurred by the families, mutual private insurance companies, and the French National Health Insurance (NHI) ;
2) FNHI: Includes costs incurred by the FNHI alone. RHF was not considered in the FNHI perspective as it is not reimbursed in France [Citation26].
Transport costs and indirect costs from care-related parental time off work were not considered.
Model inputs
Atopic manifestations and developing tolerance to cow’s milk proteins
The probabilities of AM and acquired tolerance to CMP were based on a prospective cohort study comparing AAF, EHCF+LGG, EHWF, RHF, and soy formula, over 3 years [Citation15]. To the best of our knowledge (and considering the results of a recent systematic review [Citation27]), this is the sole study providing a direct comparison between the relevant hypoallergenic formulas considered in the current analysis, and reporting on AM and probability of CMP tolerance over a 3-year follow-up.
The study protocol and methodology are described in detail in a previous publication [Citation15]. Briefly, the study on which the economic model was based recruited 365 non-breastfed infants (73 per intervention) aged less than 1 year with symptoms likely attributed to IgE-mediated CMPA. All children were symptom free at enrolment, and started on a hypoallergenic milk formula for 15–30 days by the clinician referring them to a tertiary specialist center and were receiving a diet free from cow’s milk. At baseline, IgE-mediated CMPA status was confirmed. Data on allergic manifestations were collected at follow-up visits occurring in 12 month. To check for tolerance to cow’s milk, the researchers also performed an oral food challenge, and a skin prick test to cow’s milk. Primary and secondary study outcomes for years 1 and 2 were extracted from the original publication using Engauge Digitizer software [Citation28]. The 3-year follow-up results were based on the reported outcomes. Annual probabilities of being symptom free were estimated as one minus the sum of the probabilities of any AM for that year. The efficacy parameters, except for RHF were previously published in a cost-effectiveness analysis for the UK [Citation29], and are depicted in .
Table 1. Annual probabilities of atopic manifestations, being symptom free and cumulative incidence of being tolerant to cow’s milk per comparator.
Costs and resource use
To estimate resource use in the clinical management of CMPA, a survey based on clinician experience was designed in collaboration with French clinicians, who selected six pediatricians and two pediatric gastroenterologists to participate in the survey, based on their experience managing children with CMPA. The surveyed clinicians were selected according to their distribution around the country, including those in rural and urban areas, and their practice, considering hospital and community-based clinicians. The questionnaires were sent to the clinicians in advance, allowing them to prepare their answers, being completed at a later stage with the help of a facilitator familiar with the context of the analysis. Additional clarifications were requested as appropriate. In France, general pediatricians are usually the first point of contact for children with milk allergy symptoms, being responsible for the initial management and referral to other specialties, as dictated by European guidance [Citation9]. Due to the nature of the survey used in this study, approval by an ethics committee was not required. According to the French law no. 2012–300 of 5 March 2012 [Citation30], and the Institut National de la Santé et de la Recherche Médicale (INSERM) guide [Citation31], this study was not research involving human subjects. The survey informing health resource consumption collected the anonymized opinion of experts on treatment practices, however no patient-level data was required or collected during the interview process.
Monthly requirements of hypoallergenic formula in the first 6 months were based on the EHCF+LGG formulary decision guide (876 ml per day, estimated from an average of 10 cans per month) [Citation32]. Estimated requirements after 6 months were collected in the clinician survey.
Costs associated with the diagnosis and management of the first CMPA’s symptoms were applied at baseline, to all infants. The incidence of urticaria symptoms in years 2 and 3 was assumed to be due to accidental exposure to cow’s milk or allergic reactions to other foods (as part of the atopic condition), as we had no data informing the incidence of infectious urticaria. Packed lunches and CMP-free desserts were assumed to be required 5 days per week.
The duration of the various AM was not reported by Nocerino and colleagues {Nocerino, 2021 #44} and could not be sourced elsewhere. Consequently, it was assumed that one average course of treatment/number of appointments was required to handle the annual spell of AM. Costs were obtained by multiplying the average number of resources per year by the unitary costs. Resource unitary costs were sourced from nationally available sources. Unitary costs were inflated to current values using the Consumer Price Index for Health Services [Citation33] and presented as Euros 2021. Unit costs and reimbursement rates for pediatricians and specialties’ consultations and tests were based on official public administration and FNHI data [Citation34,Citation35]. We assumed all pediatrician visits were follow-up as infants have recommended appointments by the 8th day of life. For specialist visits we assumed that only 10% were first visits. Due to the lack of real-world data, we conservatively assumed that the cost of a private sector appointment or test for physicians with uncapped fees was the same as for those with capped fees. These costs were combined with the rates of reimbursement to adjust the unit costs of a physician appointment to the different perspectives in the model. Dietitian visits were assumed to have the same cost as specialist visits and were assumed to be free to families and supported by the FNHI. The unit costs for emergency department visits and laboratory tests were based on official FNHI prices [Citation36,Citation37]. Laboratory test costs included the costs of a hemogram and biochemistry and the costs of blood collection. The cost of hospital admission was based on the costs per admission due to various nutritional disorders in children [Citation38].
Unit costs of hypoallergenic formulas were based on the average price per 100 ml of reconstituted formula, weighted by the market share per hypoallergenic formula category [Citation39]. Formula costs for FNHI and diet supplements prescribed were based on official tariffs paid according to hypoallergenic formula category [Citation40,Citation41]. Unit costs and market shares of hypoallergenic formulas are presented in Supplemental Data (Table S2). The cost of packed lunches was based on the lowest prices on large-scale retail outlets in France [Citation42]. Unit costs of prescribed medicines were based on official prices and reimbursement rates [Citation43,Citation44]. The cost of emollients without corticosteroids and spacers for inhaled medicines were based on online pharmacy prices in France. describes the unit costs used in the model and the respective sources. Estimated costs were discounted at 2.5% rate after year 1, as per the French guidelines for the economic evaluation of health care technologies [Citation12].
Table 2. Unit costs of healthcare resources and nutrition, per perspective.
Measures of effect
This analysis used the probability of acquiring cow’s milk tolerance and the absence of AM of CMPA as the main measures of effect. The probability of being free from AM was determined as one minus the sum of the probabilities of having any AM at the 3-year follow-up of the study [Citation15]. The probability of being cow’s milk tolerant used previously reported estimates [Citation15]. Time free from symptoms and time being tolerant to CMP were also analyzed, considering the 3-year time horizon since formula feeding initiation. Estimating preference-based measures of quality of life in children is a complex subject in the field of health economic evaluation, this is particularly difficult before the age of 6 years old [Citation51], therefore quality-adjusted life years (QALYs) were considered inappropriate for this analysis. Health consequences were discounted annually at a 2.5% rate [Citation12].
Sensitivity analyses
Sensitivity analyses are essential in health economic evaluation studies, in order to assess the impact of any biased parameters on the robustness of the results [Citation12,Citation52]. Clinical outcomes based on a prospective non-randomized study and resource use data based on clinicians’ survey increase the potential for uncertainty. Parameters uncertainty was explored by subjecting, all effect and cost parameters to one-way and probabilistic sensitivity analyses.
The parameters with a higher impact on the model results were summarized in a tornado diagram, based on the 95% confidence intervals of the deterministic inputs (one-way sensitivity analyses). To explore bias around the type of fees (capped or uncapped) charged by clinicians operating in the private sector, uncaptured variance in resource utilization, and country-wide variance in costs, we have conducted a scenario where total health-care costs were raised by 40%.
In probabilistic sensitivity analysis, model inputs were applied distributions in order to sample 1,000 Monte Carlo simulations. Annual probabilities of AM and being symptom free were sampled from Dirichlet distributions using events of interest and complements reported previously [Citation15]. Annual probabilities of being cow’s milk tolerant were sampled from beta distributions also by using frequency of the event of interest and complement [Citation52]. Uniform distributions were applied to costs, varying the mean estimates by 40%, as no variance measures were publicly available and unitary prices for products with the same active principle are subject to variability.
Results
Survey of clinicians
The survey elicited the average number of annual contacts with medical services required to manage CMPA and specific AM, according to children’s age, and time since formula feeding initiation. Data were collected on the frequency of pediatricians’ appointments, emergency department attendances, hospital admissions, referrals to other specialties, medical tests, medicines, and dietetic replacements. summarizes the estimated amount of health resources used in the model. The distribution of patients attending hospital appointments paying capped or uncapped clinician fees were also captured by the survey and are presented in Supplemental Data (Table S1). The clinician-reported hypoallergenic formula requirements averaged 563 ml per day from 6 to 12 months and 413 ml per day after 12 months of age.
Table 3. Estimates of resource use in the model (experts panel).
Base case
Children receiving EHCF+LGG were associated with a higher probability of being symptom free and tolerant to CMP after 3 years [Citation15]. For the overall 3-year time-horizon, EHCF+LGG was also related to an increase in time free from AM and in time tolerant to cow’s milk proteins. Children receiving EHCF+LGG were predicted to incur lower total costs from the Collective and FNHI perspectives, compared to children on EHWF or AAF. The strategy using EHCF+LGG was therefore considered dominant for all assessed outcomes, being associated with less incremental costs and more incremental effects. From the Collective perspective, with RHF being included in the analysis, EHCF+LGG remained the dominant strategy.
depicts the deterministic results of the model per considered perspective. The ratios of incremental costs to incremental benefits (symptom free or tolerance to cow’s milk) – incremental cost-effectiveness ratios (ICER), are not depicted as their negative results due to savings related to EHCF+LGG use would be misleading. A net monetary benefit was not estimated because there are no formal willingness to pay (WTP) thresholds for child per life years free from symptoms or for child tolerant to CMP outcomes.
Table 4. Base case deterministic results per symptom free and tolerance to cow’s milk, and perspective (discounted).
Base case deterministic results per symptom free and tolerance to cow’s milk, and perspective (discounted) ≫
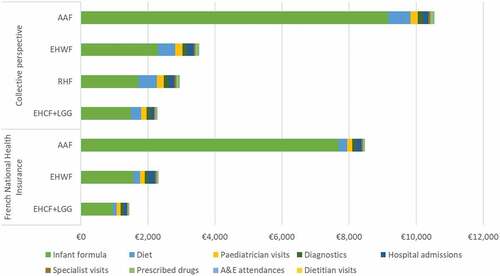
Total costs per healthcare resources are represented in and in Supplemental Data (Table S3). From the Collective perspective, hypoallergenic formulas were responsible for the largest proportion of overall costs averaging 69% across all comparators, with a minimum of 58% for RHF and maximum of 87% for AAF. Dietetic replacements options (other than formulas) were the second highest cost component, representing approximately 13% of total costs across comparators. Diagnostic tests, pediatricians’ visits and hospital admissions added up to 14% of total costs. Specialist appointments, emergency department attendances, prescribed drugs, and dietitian visits represented 4% of total costs amongst all comparators. Our analysis predicted that children receiving EHCF+LGG incurred 37%, 37%, and 44% less costs on health care and nutrition resources compared to those on RHF, EHWF, and AAF, respectively. Differences in healthcare resource use and costs are a direct consequence of the incidence of AM per formula. From the FNHI perspective, CMP free packed lunches and private dietitians’ visits were not reimbursed, so 75% of total FNHI costs are due to the infant formula (91% for AAF) and 16% are due to diagnostic tests, pediatricians’ visits and hospital admissions. Children receiving EHCF+LGG were associated with savings of 35% and 38% in FNHI costs, compared to those on EHWF and AAF.
Sensitivity analyses
One-way sensitivity analysis
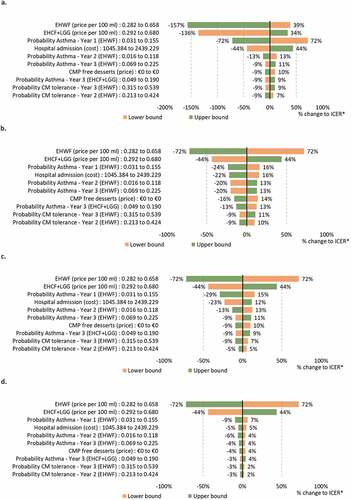
One-way sensitivity analyses were conducted by evaluating the ICERs resulting from changing the value of all model parameters (one at a time) to the lower and upper bounds of their 95% confidence interval. These analyses were summarized in tornado diagrams depicting the results for the ten most influential parameters. EHWF was chosen for sensitivity analyses presentation as it has shown to be the second-best option on all outcomes considered, after EHCF+LGG. One-way sensitivity analyses comparing EHCF+LGG to EHWF under the Collective perspective are shown in . One-way analyses for the FNHI perspective are shown in Supplemental Data (Figure S1). Bear in mind that ICERs are negative as EHCF+LGG dominates the comparator (increased benefits at lower costs). The higher the negative value (in absolute terms), more savings are estimated for using EHCF+LGG when compared to EHWF.
The model results were more sensitive to the annual probabilities of being symptom free and to the cumulative probability of acquiring tolerance to cow’s milk, according to the outcome that was live in the model. As expected, hypoallergenic formula prices also had a major impact on the results. However, varying all these parameters in the one-way analysis did not influence the model conclusions, in both the Collective and FNHI perspectives.
Scenario analyses
We have explored uncertainty around clinician fees and other cost variation across France by increasing total healthcare costs by 40% in all comparators. These has caused no change to the conclusions of the model with EHCF+LGG remaining the most cost-effective strategy from the FNHI and Collective perspectives. Increasing healthcare costs led to small variation in the overall contribution of infant formula to total costs, becoming 69% (6% less than base case) and 64% (5% less than base case), from the FNHI and Collective perspectives, respectively.
Probabilistic sensitivity analyses
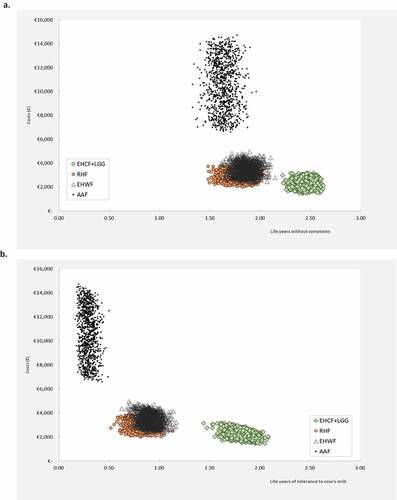
We have conducted probabilistic sensitivity analysis to explore and represent the uncertainty associated with all model inputs simultaneously. The average results of 1,000 iterations of the probabilistic sensitivity analysis were almost identical to the deterministic results. is the graphical representation of the probabilistic sampling on the cost-effectiveness plane when life years without symptoms and life years with tolerance to cow’s milk are shown from the Collective perspective. Probabilistic sampling plots for other outcomes and for the FNHI perspective are presented as Supplemental Data (Figures S2 and S3). The cost-effectiveness acceptability curves estimated for life years without symptoms and life years with tolerance to cow’s milk are shown in . EHCF+LGG was associated with the highest probability of being cost-effective. At a willingness to pay of €1,000 per life year lived without symptoms or life year with tolerance to cow’s milk, EHCF+LGG was associated with an 100% probability of being the most cost-effective strategy. This remained true for the remaining range of WTP values, from both the Collective and FNHI perspectives.
Figure 5. Cost-effectiveness acceptability curves (Collective perspective).
Discussion
The results of a recent clinical trial reporting on AM and CMPA tolerance [Citation15], showed that children receiving EHCF+LGG had a faster improvement of CMPA symptoms and earlier tolerance to cow’s milk, leading to a decrease in healthcare needs and formula consumption, compared to alternative hypoallergenic formulas. Based on our modelled evaluation, for the 3-year time horizon and using a Collective perspective, EHCF+LGG was predicted to save at least €674 per child when compared to RHF and at least €1,248 compared to EHWF or AAF, respectively. From the FNHI perspective, EHCF+LGG savings were estimated at a minimum of €875 per child, compared to the prescription of EHWF or AAF. The current cost-effectiveness analysis suggests that EHCF+LGG is the dominant strategy, providing most clinical and economic benefits.
Comparing previous economic evaluations of whey-based hydrolyzed formulas in the French setting with the current work should be done with caution due to the fundamental differences in the choice of comparators, outcomes, and methodology [Citation18,Citation19]. Comparisons to previously published cost-effectiveness analysis of hypoallergenic formulas in other countries must also take into account the different comparators and cost perspectives considered. These may not be transferable to the French setting. In 2015, EHCF+LGG was compared to EHCF alone and AFF in the perspective of the Spanish National Health Services, concluding that EHCF+LGG was associated with improved outcomes at lower costs [Citation21]. In the same year, a similar study comparing EHCF+LGG to EHCF alone, AFF, the soy formula and RHF in the Italian National Health Service perspective demonstrated the same conclusions, with EHCF+LGG being the dominant option over all the other comparators [Citation20]. In 2016, Guest and colleagues estimated the cost-effectiveness of EHCF+LGG versus EHCF alone and AFF in the perspectives of the Polish National Health Fund and parents. Though healthcare costs were reduced for the Polish National Health Fund in infants fed with EHCF+LGG, AAF provided marginally lower costs to parents [Citation22]. In 2019, Guest and Singh concluded that EHCF+LGG were associated to improved outcomes at lower costs over a 5-year time horizon from the perspective of the National Health Service in the UK [Citation23]. A recent analysis based on Nocerino and colleagues’ study compared EHCF+LGG to EHWF and soy formula in the National Health Service in the UK, having concluded for reduced healthcare costs in infants utilizing EHCF+LGG, over a 3-year period [Citation29].
Limitations of the current economic analysis are worth taking into consideration when seeking to apply the results described here into clinical practice. First, efficacy outcomes rely on the results of a non-randomized prospective study performed at a sole European country, which were then combined with resource utilization data surveyed from French clinicians. In this trial, a large number of infants were included, and arms were balanced. Results of AM’s incidence were adjusted for confounding through a binary regression model. However, tolerance to cow’s milk proteins was a secondary outcome and the difference between formulas could not be proved as statistically significant [Citation15]. Nevertheless, results of an observational study provide useful evidence on effectiveness, and a valuable insight on real-world data [Citation53]. Infants were consecutively allocated in cohorts according to the formula previously used in the 2 to 4 weeks prior to enrolment, and the most commonly used substitutive formulas were compared. Furthermore, the study results are in line with previous evidence of the effect of hypoallergenic formulas on the incidence of AM [Citation54–57] and acquisition of immune tolerance [Citation13,Citation14,Citation27,Citation54–60]. Notwithstanding, comparisons should be done with caution, as study designs, formulas used and outcomes differ. To the best of our knowledge and according to a published literature review in this topic area, there is no randomized study comparing the hypoallergenic formulas relevant for the France analysis [Citation27].
Resource estimation was obtained from six pediatricians and two gastroenterologists practicing in France, and unit costs were based on official and publicly available sources from France. Face validity of the utilized inputs were subject to scrutiny by experienced French clinicians, as no other publications were found to inform these inputs. The remaining uncertainty about the fees being paid by families accessing private medical appointments and other possible variations in healthcare resources use was explored in a scenario analysis.
Health economics is a decision science in which cost-effectiveness analysis is used to guide the efficient allocation of resources. This often requires synthesis of evidence to consider all available treatment options, however gaps in the evidence base can often exist. Despite these limitations, decisions still need to be made, hence economic modelling as described here is used to compare costs and outcomes of different options. The current analysis uses the best available evidence, modelling to fill the gaps where data does not exist or to reduce potential bias from clinical and costs data. To address evidence limitations, sensitivity analyses were performed to account for variation in outcomes and costs that could occur in clinical practice. As reported here, the findings from the sensitivity analysis support the conclusions from the base case analysis, and expected variability in outcomes for different treatments did not influence the main conclusions. Furthermore, the model assumed the time horizon of the clinical trial’s follow-up in order to avoid adding uncertainty from any extrapolation method. A previous publication has shown that longer time horizons would not change the cost-effectiveness conclusions in CMPA [Citation23].
The outcome measures evaluated in the current model were the probability of being free from AM and acquiring cow’s milk tolerance, and life years without symptoms and while tolerant to cow’s milk, rather than quality-adjusted life years (QALYs), commonly used in French economic evaluations [Citation12]. QALYs were not considered as there are several methodological questions regarding utility estimates in children under 5 years old [Citation12] and a large variation on how AM affect each infant (namely the frequency, intensity, and duration of each AM), that would compromise the utility assessment. Furthermore, AM may also impact families’ quality of life. Hence, we perceived the selected outcomes as meaningful to the clinical community and families.
According to the efficacy outcomes’ source, adverse events were not considered in the current model [Citation15]. The cost-effectiveness analysis of the clinical nutrition sequence in non-breastfed children with CMPA would also be interesting to assess in future analyses.
At last, it is also important to note that the current analysis excludes externalities of improving CMPA symptoms and of acquiring tolerance to cow’s milk. Positive externalities are expected on child development and on adulthood, but also on parents and families’ well-being.
Conclusions
This analysis compares the costs and consequences of managing CMPA in children using available hypoallergenic formulas in France based on the best available evidence, from a recently published non-randomized comparative trial. Compared to other common hypoallergenic formulas, EHCF+LGG was predicted to be the most cost-effective strategy, being associated with lower total costs, directly linked to higher oral tolerance to cow’s milk and less AM. Acknowledging the cost breakdown, along incremental benefits, allows clinicians and families to understand detailed impact on both healthcare system and out of pocket expenses, and take informed decisions. Immune tolerance is likely to positively affect child development, families’ wellbeing, and substantially reduce health care and infant formula costs. Although the results are subject to some data limitations, we do believe they are helpful to inform the choice of hypoallergenic formula for non-breastfed children in France.
Disclosure statement
No potential conflict of interest was reported by the author(s).The authors M.M. and N.P. are employees of the sponsor organisation.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Hansen K. Breastfeeding: a smart investment in people and in economies. Lancet. 2016;387(10017):416.
- Victora CG, Bahl R, Barros AJD, et al. Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet. 2016;387(10017):475–15.
- Li R, Fein SB, Chen J, et al. Why mothers stop breastfeeding: mothers’ self-reported reasons for stopping during the first year. Pediatrics. 2008;122:S69.
- Savage J, Johns CB. Food allergy: epidemiology and natural history. Immunol Allergy Clin North Am. 2015;35(1):45–59.
- Venter C, Arshad SH. Epidemiology of food allergy. Pediatr Clin North Am. 2011;58(2):327–349.
- Flom DJ, Sicherer HS. Epidemiology of cow’s milk allergy. Nutrients. 2019;11(5):1051.
- Nwaru BI, Hickstein L, Panesar SS, et al. The epidemiology of food allergy in Europe: a systematic review and meta-analysis. Allergy. 2014;69(1):62–75.
- Rona RJ, Keil T, Summers C, et al. The prevalence of food allergy: a meta-analysis. J Allergy Clin Immunol. 2007;120(3):638–646.
- Koletzko S, Niggemann B, Arato A, et al. Diagnostic approach and management of cow’s-milk protein allergy in infants and children: ESPGHAN GI Committee practical guidelines. J Pediatr Gastroenterol Nutr. 2012;55(2):221–229.
- Walsh J, Meyer R, Shah N, et al. Differentiating milk allergy (IgE and non-IgE mediated) from lactose intolerance: understanding the underlying mechanisms and presentations. Br J Gen Pract. 2016;66(649):e609–e611.
- Nowak-Wegrzyn A, Chehade M, Groetch ME, et al. International consensus guidelines for the diagnosis and management of food protein-induced enterocolitis syndrome: executive summary-workgroup report of the adverse reactions to foods committee, american academy of allergy, asthma & immunology. J Allergy Clin Immunol. 2017;139(4):1111–1126 e1114.
- de Santé HA. Methodological Guidance - Choices in methods for economic evaluation. 2020 Retrieved 23 June 2021. Available from: www.has-sante
- Berni Canani R, Nocerino R, Terrin G, et al. Formula selection for management of children with cow’s milk allergy influences the rate of acquisition of tolerance: a prospective multicenter study. J Pediatr. 2013;163(3):771–777 e771.
- Berni Canani R, Di Costanzo M, Bedogni G, et al. Extensively hydrolyzed casein formula containing Lactobacillus rhamnosus GG reduces the occurrence of other allergic manifestations in children with cow’s milk allergy: 3-year randomized controlled trial. J Allergy Clin Immunol. 2017;139(6):1906–1913 e1904.
- Nocerino R, Bedogni G, Carucci L, et al. The impact of formula choice for the management of pediatric cow’s milk allergy on the occurrence of other allergic manifestations: the atopic march cohort study. J Pediatr. 2021;232:183–191.e3.
- Weill C, Banta D. Development of health technology assessment in France. Int J Technol Assess Health Care. 2009;25(1):108–111.
- Dubromel A, Geffroy L, Aulagner G, et al. Assessment and diffusion of medical innovations in France: an overview. J Mark Access Health Policy. 2018;6(1):1458575.
- Iskedjian M, Dupont C, Spieldenner J, et al. Economic evaluation of a 100% whey-based, partially hydrolysed formula in the prevention of atopic dermatitis among French children. Curr Med Res Opin. 2010;26(11):2607–2626.
- Spieldenner J, Belli D, Dupont C, et al. Partially hydrolysed 100% whey-based infant formula and the prevention of atopic dermatitis: comparative pharmacoeconomic analyses. Ann Nutr Metab. 2011;59(1):44–52.
- Guest JF, Panca M, Ovcinnikova O, et al. Relative cost-effectiveness of an extensively hydrolyzed casein formula containing the probiotic Lactobacillus rhamnosus GG in managing infants with cow’s milk allergy in Italy. Clinicoecon Outcomes Res. 2015;7:325–336.
- Guest JF, Weidlich D, Mascunan Diaz JI, et al. Relative cost-effectiveness of using an extensively hydrolyzed casein formula containing the probiotic Lactobacillus rhamnosus GG in managing infants with cow’s milk allergy in Spain. Clinicoecon Outcomes Res. 2015;7:583–591.
- Guest JF, Weidlich D, Kaczmarski M, et al. Relative cost-effectiveness of using an extensively hydrolyzed casein formula containing the probiotic Lactobacillus rhamnosus GG in managing infants with cow’s milk allergy in Poland. Clinicoecon Outcomes Res. 2016;8:307–316.
- Guest JF, Singh H. Cost-effectiveness of using an extensively hydrolyzed casein formula supplemented with Lactobacillus rhamnosus GG in managing IgE-mediated cow’s milk protein allergy in the UK. Curr Med Res Opin. 2019;35(10):1677–1685.
- Husereau D, Drummond M, Petrou S, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. Value Health. 2013;16(2):e1–5.
- Edwards C, Younus M. Cow milk allergy. StatPearls Publishing. 2021;2021:1.
- Dupont C, Bocquet A, Tome D, et al. hydrolyzed rice protein-based formulas, a vegetal alternative in cow’s milk allergy. Nutrients. 2020;12(9):2654.
- Strozyk A, Horvath A, Meyer R, et al. Efficacy and safety of hydrolyzed formulas for cow’s milk allergy management: a systematic review of randomized controlled trials. Clin Exp Allergy. 2020;50(7):766–779.
- Mitchell MM, Winchen B, Wilms T, et al. markummitchell/engauge-digitizer: nonrelease. 2022.
- Martins R, Minshall E, Minshall E. Cost-effectiveness analysis of hypoallergenic milk formulas for the management of cow’s milk protein allergy in the UK. J Health Econ Outcomes Res. 2021;8(2):14–25.
- L’Assemblée nationale et le Sénat. LOI no 2012-300 du 5 mars 2012 relative aux recherches impliquant la personne humaine. J Off Répub Fr. 2012;2012:56.
- Amiel P. Comité d’évaluation éthique de l’inserm (CEEI), Guide de qualification des recherches en santé [A qualification guide for health research]. Inserm. 2021;2021:1.
- Mead J. Formulary decision guide: nutramigen. 2019.
- Institut National de la Statistique et des Études Économiques (Insee). Indice des prix à la consummation – base 2015 – ensemble des ménages – services de santé. 2021. Retrieved 24 June 2021. Available from: https://www.insee.fr/fr/statistiques/serie/001763845.
- Française R. Le site officiel de l’administration française: remboursement d’une consultation médicale pour un enfant. 2014.
- l’Assurance Maladie. Consultations des enfants de moins de 16 ans. 2021. Retrieved 23 June 2021. Available from: https://www.ameli.fr/assure/remboursements/rembourse/consultations/metropole#text_649
- l’Assurance Maladie. Codage des actes médicaux (CCAM). 2021. Retrieved 23 June 2021. Available from: https://www.ameli.fr/accueil-de-la-ccam/telechargement/version-actuelle/index.php
- l’ Assurance Maladie. Aide au Codage. 2021 Retrieved 23 June 2021. Available from: https://www.aideaucodage.fr/ccam
- ScanSanté. Référentiel national de cout des prises en chanrge (ENC). 2021 Retrieved 23 June 2021. Available from: https://www.scansante.fr/applications/enc-mco
- iQVIA Data on file. Milk formula unit costs and market shares. 2021.
- Ministère des affaires sociales et de la santé. Avis relatif à la baisse de certains produits et prestations visés à l’article L. 165-1 du code de la securité sociale. J Off Répub Fr. 2016;114.
- Ministère des solidarités et de la santé. Avis relatif à la tarification des denrées alimentaires destinées à des fins médicales visées à lárticle L. 165-1 du code de la securité sociale. J Off Répub Fr. 2021.
- Carrefour. 2021. Retrieved 18 June 2021. Available from: https://www.carrefour.fr
- Comité Economique des Produits de Santé. Prix des Medicaments. 2021. Retrieved 30 June 2021. Available from: http://medicprix.sante.gouv.fr/medicprix/
- l’Assurance Maladie. Base des Médicaments et Informations Tarifaires. 2021. 18 June 2021. Available from: http://www.codage.ext.cnamts.fr/codif/bdm_it/index.php?p_site=AMELI
- CNAMTS. Les actes de biologie médicale: analyse des dépenses en 2008 et 2009. Points Rep. 2010;2010:33.
- Shop Pharmacie. 2021. Retrieved18 June 2021. Available from: https://www.shop-pharmacie.fr
- Cocooncenter - Parapharmacie en ligne. 2021. Retrieved18 June 2021. Available from: https://www.cocooncenter.com/
- Pharmacie Veau. Available from: 2021. Retrieved18 June 2021. Available from: https://www.pharmacieveau.fr/
- DoctiPharma by DocMorris. 2021. Retrieved18 June 2021. Available from: https://www.doctipharma.fr/
- Ministère des Solidarités et de la Santé. Avis relatif à la tarification des denrées alimentaires destinées à des fins médicales spéciales visées à l’article L. 165-1 du code de la sécurité sociale. J Off Répub Fr. 2021.
- NICE Decision Support Unit. Technical support document 8: an introduction to measurement and valuation of health for NICE submissions. 2011.
- Briggs A, Sculpher M, Claxton K. Decision modelling for health economic evaluation. Oxford, UK: Oxford University Pres; 2006.
- Hackshaw A. A concise guide to clinical trials. Oxford, UK: John Wiley & Sons; 2011.
- von Berg A, Filipiak-Pittroff B, Krämer U, et al. The German Infant Nutritional Intervention Study (GINI) for the preventive effect of hydrolyzed infant formulas in infants at high risk for allergic diseases. Design and selected results. Allergol Select. 2017;1(1):28–38.
- von Berg A, Filipiak-Pittroff B, Krämer U, et al. Preventive effect of hydrolyzed infant formulas persists until age 6 years: long-term results from the German Infant Nutritional Intervention Study (GINI). J Allergy Clin Immunol. 2008;121(6):1442–1447.
- von Berg A, Koletzko S, Filipiak-Pittroff B, et al. Certain hydrolyzed formulas reduce the incidence of atopic dermatitis but not that of asthma: three-year results of the German Infant Nutritional Intervention Study. J Allergy Clin Immunol. 2007;119(3):718–725.
- von Berg A, Koletzko S, Grübl A, et al. The effect of hydrolyzed cow’s milk formula for allergy prevention in the first year of life: the German Infant Nutritional Intervention Study, a randomized double-blind trial. J Allergy Clin Immunol. 2003;111(3):533–540.
- Baldassarre ME, Laforgia N, Fanelli M, et al. Lactobacillus GG improves recovery in infants with blood in the stools and presumptive allergic colitis compared with extensively hydrolyzed formula alone. J Pediatr. 2010;156(3):397–401.
- Berni Canani R, Nocerino R, Terrin G, et al. Effect of lactobacillus GG on tolerance acquisition in infants with cow’s milk allergy: a randomized trial. J Allergy Clin Immunol. 2012;129(2):580–582, 582.e581–585.
- Sánchez-Valverde F, Etayo V, Gil F, et al. Factors associated with the development of immune tolerance in children with cow’s milk allergy. Int Arch Allergy Immunol. 2019;179(4):290–296.