ABSTRACT
Background
The evidence base of tisagenlecleucel is uncertain.
Objective
To evaluate the cost-effectiveness of tisagenlecleucel. To conduct expected value of perfect information (EVPI) and partial EVPI (EVPPI) analyses.
Study Design
A three-state partitioned survival model. A short-term decision tree partitioned patients in the tisagenlecleucel arm according to infusion status. Survival was extrapolated to 5 years; general population mortality with a standardised mortality ratio was then applied. EVPI and EVPPI were scaled up to population according to the incidence of the decision.
Setting
Irish healthcare payer.
Participants
Patients with relapsed/refractory diffuse large B-cell lymphoma (R/R DLBCL).
Interventions
Tisagenlecleucel versus Salvage Chemotherapy (with or without haematopoietic stem cell transplant).
Main Outcome Measure
Incremental cost-effectiveness ratio (ICER). Population EVPI and EVPPI.
Results
At list prices, the ICER was €119,509 per quality-adjusted life year (QALY) (incremental costs €218,092; incremental QALYs 1.82). Probability of cost-effectiveness, at a €45,000 per QALY threshold, was 0%. Population EVPI was €0.00. Population EVPI, at the price of tisagenlecleucel that reduced the ICER to €45,000 per QALY, was €3,989,438. Here, survival analysis had the highest population EVPPI (€1,128,053).
Conclusion
Tisagenlecleucel is not cost-effective, versus salvage chemotherapy (with or without haematopoietic stem cell transplant), for R/R DLBCL in Ireland. At list prices, further research to decrease decision uncertainty may not be of value.
Introduction
Diffuse large B-cell lymphoma (DLBCL) accounts for 25% to 30% of all non-Hodgkin’s lymphoma diagnoses [Citation1]. It displays an aggressive course [Citation2]. In Ireland, the 5-year survival of patients with newly diagnosed DLBCL is 62% [Citation3]. Patients with refractory disease, or those who experience multiple relapses, have poor prognosis [Citation4]. Median overall survival (OS) has ranged from 4.4 to 10 months [Citation5–7].
Tisagenlecleucel (a CAR T-cell therapy) received European Medicines Agency (EMA) conditional marketing authorisation (2018) for the treatment of adult patients with relapsed or refractory DLBCL, after two or more lines of systemic therapy (R/R DLBCL) [Citation8]. CAR T-cell therapy has an innovative mechanism of action, whereby T-cells from a patient are genetically engineered to express chimeric antigen receptors (CARs). These CARs allow the genetically modified T-cells (CAR T-cells) to recognise and eliminate CD19 antigen-expressing cells [Citation9]. Tisagenlecleucel has been proposed by some to be potentially curative [Citation10]. However, much uncertainty exists in the evidence base. Marketing authorisation was granted based on a single-arm trial, with short duration of follow-up; JULIET [Citation11]. The long-term survival benefit of tisagenlecleucel may not be realised in clinical practice. Payers are at financial risk due to the high upfront cost.
Aim
The aim of this study was to evaluate the cost-effectiveness of tisagenlecleucel, versus salvage chemotherapy with or without haematopoietic stem cell transplant (HSCT) (henceforth ‘salvage chemotherapy’), for R/R DLBCL in the Irish healthcare setting. The value of conducting further research to investigate uncertainties in the model was assessed by expected value of perfect information (EVPI) and partial EVPI (EVPPI) analyses.
Model
Model structure
Short-term decision tree
The model comprised a short-term decision tree (tisagenlecleucel only) and a long-term partitioned survival model (both arms) [Citation12]. The decision tree captured costs and outcomes associated with the tisagenlecleucel pre-treatment phase. All patients in the tisagenlecleucel arm entered the decision tree, underwent leukapheresis, and subsequently progressed to one of three pathways (Supplementary Figure S1):
N1: proceeded to tisagenlecleucel infusion (69% of patients [Citation13]).
N2: did not proceed to infusion due to manufacturing failure, adverse event (AE), or physician/patient decision (19% of patients [Citation13]). Instead, these patients were assumed to receive salvage chemotherapy.
N3: did not proceed to infusion due to death (12% of patients [Citation13]). These did not receive any further active treatment.
Partitioned survival model
The partitioned survival model simulated the progression of patients through three, mutually exclusive health states: progression-free survival, progressed disease, and death (Supplementary Figure S2). Most patients with DLBCL are expected to relapse within 24–60 months post-treatment [Citation14–16]. It was, therefore, assumed that patients who were alive after 60 months in either arm were long-term survivors. These were subject to age- and sex-matched general population mortality with a standardised mortality ratio (1.36) applied [Citation16].
Cycle length was 1 month (30.4 days); a half-cycle correction was applied. A lifetime horizon of 44 years was employed. A discount rate of 4% was applied to costs and outcomes [Citation17].
Population
The population was aligned with the EMA licensed population of tisagenlecleucel [Citation8]. Starting age was 56 years, 61% were male, body weight was 78.7 kg and body surface area was 1.92 m2 [Citation11,Citation18–20].
Intervention
The intervention was tisagenlecleucel, administered at the EMA licensed dose and modelled as a single-dose intervention [Citation8].
Comparator
There is no universal routine care for patients with R/R DLBCL in Ireland. R-GDP (rituximab, gemcitabine, dexamethasone, cisplatin; with or without HSCT) was assumed to represent salvage chemotherapy [Citation21,Citation22]. Dosing was in line with published chemotherapy regimens [Citation23]. Based on clinical opinion in Ireland, patients were assumed to receive three cycles. Also, based on clinical opinion, 15% of patients are expected to proceed to HSCT (usually allogeneic stem cell transplant) following treatment with R-GDP.
Perspective
The perspective was that of the healthcare payer, the Health Service Executive (HSE) in Ireland [Citation17]. Direct medical costs were included.
Model inputs
Efficacy data
Treatment effectiveness was based on the effect on OS and progression-free survival (PFS). Efficacy data, identified by systematic literature review, were derived from JULIET (tisagenlecleucel) [Citation13,Citation18], and CORAL Extension 1 (proxy data for R-GDP) [Citation5]. JULIET (n = 115) is a phase II, single-arm trial, which evaluated the efficacy of tisagenlecleucel in the population of interest here [Citation13]. CORAL Extension 1 is an observational study, reporting outcomes of a cohort (n = 203) receiving third-line therapy for R/R DLBCL [Citation5]. Patients in CORAL Extension 1 received a range of salvage chemotherapy regimens. Due to the small number of patients receiving each salvage chemotherapy regimen, a comparison of tisagenlecleucel versus pooled data, derived from all salvage chemotherapy regimens, was deemed most appropriate. Further details of the trials are provided in Supplementary Table S1.
Individual patient-level data (IPD) from published Kaplan–Meier curves of OS and PFS were reconstructed, using Digitzelt software and the algorithm by Guyot et al. [Citation24,Citation25]. Due to the single-arm nature of the trials, and lack of publicly available raw IPD, a naïve comparison was conducted.
Extrapolation of survival data was conducted, in line with NICE Decision Support Unit Guidance (technical support document 14 [Citation26]). Standard parametric (Gompertz, exponential, Weibull, log-logistic, log-normal, generalised gamma) extrapolation models were explored [Citation26]. Due to the innovative mechanism of tisagenlecleucel and the potential for complex hazard functions [Citation27], flexible cubic spline models (one-, two-, and three-knot spline models across all scales), and mixture cure models were also explored. Survival models were fitted individually to the treatment arms. The best fitting model was selected based on AIC and BIC statistics (Supplementary Tables S3–S6), visual fit (Supplementary Figures S3-S5), and clinical plausibility [Citation26].
Overall survival
The two-knot (hazard) spline model was chosen to extrapolate JULIET OS data. This most closely aligned with observed data [Citation28] and provided good statistical and visual fit.
For CORAL Extension 1, separate Kaplan-Meier OS curves were presented for patients who did and did not receive HSCT. Separate extrapolation models were fitted to these curves. To model OS of the overall population (those with and without HSCT), a weighted OS curve combining extrapolations from the separate Kaplan–Meier curves was generated. The weight applied corresponded to the expected rate of HSCT in Irish clinical practice (15%). The Gompertz model was chosen to extrapolate the CORAL Extension 1 (with and without HSCT) data.
Progression-free survival
The one-knot (hazard) spline model was chosen to extrapolate JULIET PFS data.
PFS was not reported for CORAL Extension 1. Thus, PFS was estimated from OS by assuming the cumulative hazard function for PFS was proportional to the cumulative hazard function for OS [Citation20,Citation29]. The hazard ratio between PFS and OS (0.65) was based on the mean cumulative hazard ratio from the CORAL, phase III, randomised trial [Citation30,Citation31], and was identified through the literature [Citation20,Citation29]. The overall cumulative hazard was applied to the CORAL Extension 1 OS data to generate PFS. The PFS predicted for salvage chemotherapy was therefore, contingent upon the model applied to the OS data.
After month 60, cumulative survival probabilities for PFS were assumed to flatten up to the point at which PFS met OS. Death due to progression only occurred within the first 60 months of the model in both arms, as patients alive after 60 months were assumed to be long-term survivors. PFS could not exceed OS at any point.
Utility inputs
Utility data were derived through systematic literature review (Supplementary Table S7). Health-state utility data were collected during JULIET, using the SF-36 and mapped to the EQ-5D-3 L, with the UK valuation set applied [Citation29,Citation32]. Patients alive after 60 months were assumed to have utility equivalent to that of the progression-free survival state. Disutility associated with pre-treatment procedures, intensive care unit (ICU) admission, febrile neutropenia, pancytopenia, and HSCT were accounted for (). An age adjustment was applied, using the multiplicative approach [Citation40].
Table 1. Key input parameters of cost-utility model of tisagenlecleucel versus salvage chemotherapy.
Cost inputs
Irish cost data were used, where available. Where necessary, costs were inflated to 2020 using the Consumer Price Index for health [Citation41], and converted to Euro using purchasing power parities [Citation42].
Training
As per EMA marketing authorisation, healthcare professionals who prescribe, dispense, or administer tisagenlecleucel, require training [Citation11]. An associated per patient cost was included in the tisagenlecleucel arm (Supplementary Tables S8–S9).
Tisagenlecleucel-specific pre-treatment
In the tisagenlecleucel arm, all patients incurred the cost of leukapheresis and cryopreservation. Bridging chemotherapy (one cycle) and lymphodepleting chemotherapy (one cycle) were received by 90% and 93% of patients (who received infusion), respectively. This was informed by JULIET [Citation13,Citation18].
Drug acquisition
Total drug acquisition costs for tisagenlecleucel and salvage chemotherapy (R-GDP) are presented in .
Administration and hospitalisation
The cost of outpatient administration of bridging chemotherapy was accounted for. The duration of hospitalisation (including lymphodepleting chemotherapy) for patients receiving tisagenlecleucel is expected to be approximately 3 weeks, as per clinical opinion in Ireland. Different costs were sourced from the Irish DRG List and applied based on the intensity of resource use. The cost of hospitalisation represented a mean length of stay of 22.3 days [Citation36]. Patients are required to remain within 2 h of travel of the hospital for at least 4 weeks following infusion [Citation11]. It was arbitrarily assumed that 50% of patients required hospital-associated patient apartments for 6 days and that the remaining patients lived nearby.
An administration cost was applied to account for outpatient administration of salvage chemotherapy.
Initiation and monitoring
All tisagenlecleucel initiation and monitoring costs were assumed to be accounted for in the cost of hospitalisation. Outpatient initiation and monitoring costs were included in the salvage chemotherapy arm.
Health-state specific follow-up costs were applied to patients in the progression-free survival and progressed disease states [Citation43,Citation44]. Patients who were alive at 61 months incurred the cost of progression-free survival Month 61 onwards, regardless of health state.
Adverse events
Tisagenlecleucel-specific AEs included cytokine release syndrome (CRS), B-cell aplasia, febrile neutropenia, pancytopenia, and non-CRS ICU admission. These were informed by JULIET [Citation11,Citation13,Citation18]. Other AEs, in the tisagenlecleucel arm, were assumed to be captured by the cost of hospitalisation. For salvage chemotherapy, grade ≥3 AEs occurring in 5% or greater of the population were included [Citation45]. Online Supplementary Material 1.6.4 provides further detail.
Haematopoietic stem cell transplant
HSCT procedure [Citation36] and follow-up costs [Citation38] were included for the 15% of patients in the salvage chemotherapy arm who received HSCT.
Terminal care
A once-off per-patient cost associated with terminal care was applied to patients upon entering the death state [Citation39].
Key input parameters
Key input parameters are presented in .
Model outputs
Deterministic ICER
The base case analysis considered the incremental cost-effectiveness ratio (ICER), calculated from deterministic costs and deterministic quality-adjusted life years (QALYs), using standard decision rules [Citation17]. In Ireland, most drugs that have been reimbursed to date have been considered under a cost-effectiveness threshold of €45,000 per QALY [Citation46,Citation47]. This threshold was considered here.
Probabilistic ICER and scatterplot
Probabilistic sensitivity analysis (PSA) was conducted; parameters were varied according to appropriate distributions (). Results were generated using Monte Carlo Simulation (5,000 iterations).
A scatterplot of incremental costs and QALYs, generated from each PSA iteration, was constructed to illustrate the degree of uncertainty surrounding the estimates. The mean probabilistic ICER was estimated.
Cost-effectiveness acceptability curve
For each PSA iteration, the expected net monetary benefit (NMB) for tisagenlecleucel and salvage chemotherapy was estimated. From the NMB values, the probabilities of each treatment being cost-effective over a range of thresholds (€0.00 per QALY to €350,000 per QALY) were plotted, producing a cost-effectiveness acceptability curve.
One-way sensitivity analysis
One-way sensitivity analysis (OWSA) of all parameters was performed to determine the impact on the deterministic ICER of changes to individual parameters. Upper and lower bounds of the 95% confidence interval for point estimates were used, where available. Otherwise, point estimates were varied ±25%. A tornado plot was constructed, illustrating the impact of the 10 most influential parameters.
Scenario analysis
Several scenario analyses were conducted to assess the impact on the deterministic ICER of employing alternative, plausible assumptions.
Price analysis
An analysis was conducted (using the ‘Goal Seek’ function in Microsoft Excel®) to determine the decrease in the list price of tisagenlecleucel that would be required for the ICER to meet the €45,000 per QALY threshold.
Expected value of perfect information and partial expected value of perfect information
EVPI represents the estimated value of eliminating uncertainty in the model. EVPPI identifies the parameters whose uncertainty drives decision uncertainty, allowing further research to be prioritised [Citation48]. EVPI was calculated using 5,000 iterations of the PSA and over a range of thresholds. EVPPI was estimated using the Gaussian process regression approach [Citation49,Citation50]. EVPPI was calculated for the parameter categories: utility values, survival analysis, hospitalisation and monitoring costs, AE costs, and HSCT. Estimates were scaled up to population according to the incidence of the decision (mean of 36 patients per year) [Citation17]. A technology time horizon of 10 years was assumed [Citation27]. A discount rate of 4% was applied. Population EVPI estimates were plotted over a range of thresholds, to construct a population EVPI curve.
Results
Deterministic results
Deterministic model outcomes are presented in . At list prices, tisagenlecleucel was not cost-effective, versus salvage chemotherapy, at the €45,000 per QALY threshold.
Table 2. Deterministic, probabilistic, and scenario analysis results of the incremental analysis of cost-effectiveness of tisagenlecleucel versus salvage chemotherapy.
Probabilistic results
Expected incremental costs and incremental QALYs are presented in a scatterplot in . Mean expected costs and QALYs are presented in .
Figure 1. Scatterplot of incremental costs and incremental QALYs from probabilistic sensitivity analysis of tisagenlecleucel versus salvage chemotherapya.
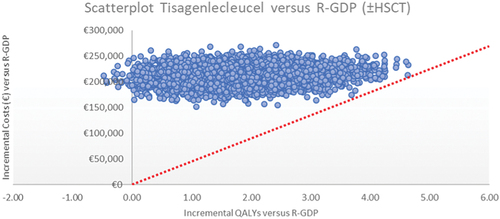
The cost-effectiveness acceptability curve is presented in Supplementary Figure S6. At the €45,000 per QALY threshold, there was a 0% probability that tisagenlecleucel was cost-effective.
One-way sensitivity analysis
Outcomes of the OWSA are presented in Supplementary Figure S7. The main drivers in the model were the discount rate on outcomes, tisagenlecleucel infusion cost, and the progression-free survival state utility value.
Scenario analysis
Results of scenario analysis are presented in and Supplementary Table S24.
Price analysis
A 65% decrease (including 5.5% rebate) on the tisagenlecleucel list price was required to reduce the deterministic ICER to the €45,000 per QALY threshold. The probability of cost-effectiveness here was 54%.
Expected value of perfect information
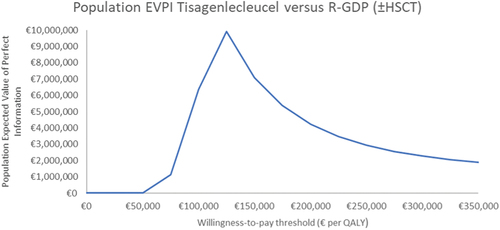
At the €45,000 per QALY threshold, the 10-year population EVPI was €0.00. Population EVPI, over a range of thresholds, is depicted in .
Figure 2. Ten-year population EVPI, over various willingness-to-pay thresholds, of tisagenlecleucel versus salvage chemotherapya.
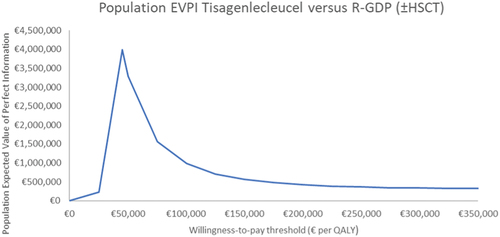
The population EVPI analysis was re-run at the price that reduced the ICER of tisagenlecleucel to €45,000 per QALY (€104,702). At this price and threshold, the 10-year population EVPI was €3,989,438 ().
Figure 3. Ten-year population EVPI, over various willingness-to-pay thresholds, of tisagenlecleucel (price that reduced the ICER to €45,000 per QALY) versus salvage chemotherapya.
Partial expected value of perfect information
At list price, EVPPI was not estimated (EVPI was €0.00).
The population EVPPI analysis was run at the price that reduced the ICER of tisagenlecleucel to €45,000 per QALY. At this threshold, survival analysis parameters had the highest population EVPPI (€1,128,053). Utility values had the second highest population EVPPI (€905,809), followed by hospitalisation and monitoring costs (€718,740), HSCT (€668,497), and AE costs (€62,932). Online Supplementary Material 1.10 depicts the value of uncertainty associated with each category.
Discussion
Deterministic and probabilistic results
At list prices, tisagenlecleucel is not cost-effective, versus salvage chemotherapy, at the €45,000 per QALY threshold. The cost-effectiveness acceptability curve indicated that the probability of cost-effectiveness of tisagenlecleucel will exceed that of salvage chemotherapy at thresholds above €117,000 per QALY. As some PSA iterations lie in the north-west quadrant (more costly, less effective), the probability of cost-effectiveness of tisagenlecleucel will not reach 100% at any threshold.
Uncertainty in the evidence base of tisagenlecleucel translates to uncertainty in cost-effectiveness. Caution should be exercised in interpretation of results presented here, as uncertainty associated with the naïve comparison is not adequately captured by OWSA, PSA and EVPI analyses.
One-way sensitivity and scenario analyses
The sensitivity of the model to the discount rate on outcomes reflects the extent to which outcomes are accrued over the long term in the model. The model was less sensitive to changes in the discount rate on costs. This illustrates the magnitude of the high upfront costs relative to the long-term QALY gain of tisagenlecleucel.
Scenario analysis highlighted the impact of changing the time-point (post-treatment) at which patients are considered long-term survivors. Changing this time-point had greater impact on QALY gain for tisagenlecleucel. The impact on incremental costs was negligible. These scenarios illustrate the reliance of the cost-effectiveness of tisagenlecleucel on the uncertain assumption of a time-point of long-term survival. Uncertainty in long-term survival is further highlighted by the sensitivity of the model to alternative extrapolation models.
OWSA indicated that the progression-free survival state utility value was a driver of cost-effectiveness. This may partly be due to the fact that all patients alive after 60 months were assumed to have health-related quality of life (HRQOL) of the progression-free survival state. The majority of QALY gains in the tisagenlecleucel arm were driven by QALYs accrued in the extrapolation of survival. HRQOL of patients considered to be long-term survivors is a key area of uncertainty [Citation54]. The model was also sensitive to employing utility values, derived from the ZUMA-1 trial (axicabtagene ciloleucel, a CAR T-cell therapy, for R/R DLBCL), for the progression-free survival and progressed disease states. Utility values derived from JULIET (base case) and ZUMA-1 (scenario analysis) are subject to limitations in that they were derived from small patient populations and are based on low numbers of observations [Citation29,Citation32,Citation55]. This reflects the challenges of collecting HRQOL data in patients with a rare disease at an advanced stage, and limits the generalisability of these data to patients in clinical practice.
Considering the uncertainty in cost-effectiveness here, performance-based risk-sharing agreements may be an appropriate approach to managing the associated financial risk. This shares the distribution of financial risk between the payer and Pharma-Applicant.
Expected value of perfect information
Population EVPI indicated that further research to decrease decision uncertainty at the €45,000 per QALY threshold may not be of value. At the population EVPI peak (threshold of approximately €125,000 per QALY), the probability of cost-effectiveness of tisagenlecleucel was 56%. As the threshold increased beyond this, probability of cost-effectiveness increased and population EVPI decreased (given the corresponding consequences of resolving decision uncertainty decreased) [Citation27]. Population EVPPI analysis was not conducted, as EVPI was €0.00. Notably, EVPI analysis examines uncertainty in parameters. Structural uncertainty, associated with the naïve comparison, was not captured.
Re-running the EVPI analysis at the price that generated an ICER of €45,000 per QALY, increased population EVPI considerably. Here, the cost of further research should not exceed €3,989,438. Population EVPPI indicated that if additional research to reduce uncertainty is conducted, research investigating survival analysis should be prioritised. The results of EVPPI analysis can be used to inform performance-based risk-sharing agreements.
Comparison with the literature
The National Centre for Pharmacoeconomics, Ireland (www.ncpe.ie) evaluated a Pharma-Applicant HTA of tisagenlecleucel for this indication (perspective of the HSE). Similar to our findings, tisagenlecleucel was not deemed cost-effective (versus salvage chemotherapy) [Citation21].
Only one identified study constructed a decision tree to model the costs and outcomes of patients who did not proceed to infusion with tisagenlecleucel in JULIET [Citation10]. Published studies, which do not account for these patients may be biased [Citation56,Citation57]. No studies identified in the literature conducted EVPI or EVPPI analyses.
Some studies employed the SCHOLAR-1 data to inform efficacy of the comparator arm [Citation10,Citation56]. These data were not used in the research here, mainly due to the poor prognosis of patients included in SCHOLAR-1. Publications, elsewhere in the literature, urge caution in the use of SCHOLAR-1 as a benchmark for prospective trials in DLBCL [Citation58,Citation59]. The approach used to model survival in the comparator arm of this model is in line with that employed elsewhere [Citation34,Citation60].
Limitations
Due to the lack of direct evidence or sufficient information to account for confounders, this analysis is underpinned by a high degree of uncertainty. The lack of direct evidence on PFS for salvage chemotherapy (CORAL Extension 1) was a notable limitation, adding further uncertainty to results. Trial designs that leverage external data have been proposed to have utility in instances where a randomised controlled trial may be deemed infeasible. Here, an external data source (real-world data, for example) can be used to create a comparator arm. Treatment effect can then be inferred by using adjustment methods, to account for differences in patient profiles between the external control group and the experimental arm. However, such approaches require patient-level data, which may not always be available [Citation61].
In the absence of detailed data on the outcomes of patients who did not proceed to infusion in JULIET, several assumptions were required. It is unclear if these truly reflect clinical practice.
The time horizon, employed in the EVPI analysis, was an arbitrary choice. In the absence of evidence, this assumption was a necessary one. Even if data are available to inform the time horizon, by means of evidence or a formal prior distribution, it will still remain a proxy [Citation62].
Conclusion
At list prices, tisagenlecleucel was not cost-effective, versus salvage chemotherapy, at the €45,000 per QALY threshold. Clinical evidence supporting the model was highly uncertain. EVPI analysis indicated that additional research to reduce decision uncertainty may not be of value. However, such analyses do not capture uncertainty associated with the naïve comparison. Caution is therefore warranted in the interpretation of results. Performance-based risk-sharing agreements may be valuable in mitigating against the financial risk associated with tisagenlecleucel.
Author contributions
All authors contributed to the study design and conception. Data analysis was performed by Niamh Carey. The initial draft of the manuscript was prepared by Niamh Carey. All authors commented on the manuscript. All authors read and approved the final manuscript.
Supplemental Material
Download MS Word (500.2 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary data
Supplemental data for this article can be accessed online at https://doi.org/10.1080/20016689.2023.2166375.
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
Additional information
Funding
References
- Chaganti S, Illidge T, Barrington S, et al. Guidelines for the management of diffuse large B-cell lymphoma. Br J Haematol. 2016;174(1):43–12. DOI:10.1111/bjh.14136
- Friedberg JW, Fisher RI. Diffuse large B-cell lymphoma. Hematol Oncol Clin North Am. 2008;22(5): 941–952. ix.
- National Cancer Registry Ireland (NCRI). Number of cases diagnosed, incidence rates, projected case counts and survival for diffuse large B-cell lymphoma. 2019.
- Feugier P, Van Hoof A, Sebban C, et al. Long-term results of the R-CHOP study in the treatment of elderly patients with diffuse large B-cell lymphoma: a study by the groupe d’Etude des lymphomes de l’adulte. J Clin Oncol. 2005;23(18):4117–4126. DOI:10.1200/JCO.2005.09.131
- Den Neste E V, Schmitz N, Mounier N, et al. Outcome of patients with relapsed diffuse large B-cell lymphoma who fail second-line salvage regimens in the International CORAL study. Bone Marrow Transplant. 2016;51(1):51–57. DOI:10.1038/bmt.2015.213
- Den Neste E V, Schmitz N, Mounier N, et al. Outcomes of diffuse large B-cell lymphoma patients relapsing after autologous stem cell transplantation: an analysis of patients included in the CORAL study. Bone Marrow Transplant. 2017;52(2):216–221. DOI:10.1038/bmt.2016.213
- Crump M, Neelapu SS, Farooq U, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood. 2017;130(16):1800–1808. DOI:10.1182/blood-2017-03-769620
- Kymriah 1.2x106 - 6x108 cells dispersion for infusion: summary of product characteristics 2021. Available from: https://www.medicines.ie/medicines/kymriah-1-2-x-106-6-x-108-cells-dispersion-for-infusion-34945/spc.
- Sterner RC, Sterner RM. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 2021;11(4):69.
- Wakase S, Teshima T, Zhang J, et al. Cost effectiveness analysis of tisagenlecleucel for the treatment of adult patients with relapsed or refractory diffuse large B cell lymphoma in Japan. Transplant Cell Ther. 2021;27(6):506.e1–506.e10. DOI:10.1016/j.jtct.2021.03.005
- European Medicines Agency (EMA). Kymriah: EPAR -public assessment report. 2018.
- Institute for Clinical and Economic Review (ICER). Chimeric antigen receptor T-cell therapy for B-cell cancers: effectiveness and value. 2018.
- Schuster SJ, Bishop MR, Tam C, et al. Sustained disease control for adult patients with relapsed or refractory diffuse large B-cell lymphoma: an updated analysis of juliet, a global pivotal phase 2 trial of tisagenlecleucel. Blood. 2018;132(Supplement 1):1684. DOI:10.1182/blood-2018-99-115252
- Maurer MJ, Ghesquières H, Jais J-P, et al. Event-free survival at 24 months is a robust end point for disease-related outcome in diffuse large B-cell lymphoma treated with immunochemotherapy. J Clin Oncol. 2014;32(10):1066–1073. DOI:10.1200/JCO.2013.51.5866
- Howlader N, Mariotto AB, Besson C, et al. Cancer-specific mortality, cure fraction, and noncancer causes of death among diffuse large B-cell lymphoma patients in the immunochemotherapy era. Cancer. 2017;123(17):3326–3334. DOI:10.1002/cncr.30739
- Jakobsen LH, Bøgsted M, Brown PN, et al. Minimal loss of lifetime for patients with diffuse large B-cell lymphoma in remission and event free 24 months after treatment: a danish population-based study. J Clin Oncol. 2017;35(7):778–784. DOI:10.1200/JCO.2016.70.0765
- Health Information and Quality Authority (HIQA). Guidelines for the Economic Evaluation of Health Technologies in Ireland. Dublin: HIQA: 2020.
- Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2018;380(1):45–56. DOI:10.1056/NEJMoa1804980
- Liu R, Oluwole OO, Diakite I, et al. Cost effectiveness of axicabtagene ciloleucel versus tisagenlecleucel for adult patients with relapsed or refractory large B-cell lymphoma after two or more lines of systemic therapy in the United States. J Med Econ. 2021;24(1):458–468.
- The Canadian Agency for Drugs and Technologies in Health (CADTH). Tisagenlecleucel for relapsed/refractory diffuse large B-cell lymphoma. Economic Review Report. Ottawa: CADTH; 2019.
- National Centre for Pharmacoeconomics (NCPE). Tisagenlecleucel (Kymriah) for the treatment of adult patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) after two or more lines of systemic therapy: summary. 2019.
- National Centre for Pharmacoeconomics (NCPE). Axicabtagene ciloleucel for the treatment of adult patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) and primary mediastinal large B-cell lymphoma (PMBCL) after two or more lines of systemic therapy: technical summary. 2019.
- National Cancer Control Programme (NCCP). Rituximab, Gemcitabine, Dexamethasone and CISplatin ((R)-GDP) Therapy. 2019.
- Guyot P, Ades AE, MJNM O, et al. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol. 2012;12(1):9.
- Digitizelt Digitizer S. 2020 [cited 2020 Apr 04]. Available from: https://www.digitizeit.xyz/.
- Latimer N. NICE DSU Technical Support Document 14: Undertaking survival analysis for economic evaluations alongside clinical trials - extrapolation with patient-level data. University of Sheffield: Decision Support Unit; 2011. Available from: http://nicedsu.org.uk/.
- Hettle R, Corbett M, Hinde S, et al. The assessment and appraisal of regenerative medicines and cell therapy products: an exploration of methods for review, economic evaluation and appraisal. Health Technol Assess. 2017;21(7):1–204. DOI:10.3310/hta21070
- Jaeger U, Bishop MR, Salles G, et al. Myc expression and tumor-infiltrating T cells are associated with response in patients (pts) with relapsed/refractory diffuse large B-Cell lymphoma (r/r DLBCL) treated with tisagenlecleucel in the juliet trial. Blood. 2020;136(Supplement 1):48–49. DOI:10.1182/blood-2020-137045
- Norwegian Medicines Agency (NoMA). Single technology assessment: tisagenlecleucel (Kymriah) for the treatment of second or later relapsed/refractory diffuse large B-cell lymphoma (DLBCL). 2019.
- Gisselbrecht C, Schmitz N, Mounier N, et al. Rituximab maintenance therapy after autologous stem-cell transplantation in patients with relapsed CD20+ diffuse large B-cell lymphoma: final analysis of the collaborative trial in relapsed aggressive lymphoma. J Clin Oncol. 2012;30(36):4462–4469. DOI:10.1200/JCO.2012.41.9416
- Gisselbrecht C, Glass B, Mounier N, et al. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J Clin Oncol. 2010;28(27):4184–4190. DOI:10.1200/JCO.2010.28.1618
- Maziarz RT, Waller EK, Jaeger U, et al. Patient-reported long-term quality of life after tisagenlecleucel in relapsed/refractory diffuse large B-cell lymphoma. Blood Adv. 2020;4(4):629–637. DOI:10.1182/bloodadvances.2019001026
- Guadagnolo BA, Punglia RS, Kuntz KM, et al. Cost-effectiveness analysis of computerized tomography in the routine follow-up of patients after primary treatment for Hodgkin’s disease. J Clin Oncol. 2006;24(25):4116–4122.
- Corbett M, Duarte A, Walker S, et al. Tisagenlecleucel for treating relapsed or refractory diffuse large B-cell lymphoma: a single technology appraisal. University of York: University of York Technology Assessment Group; 2018.
- Lloyd A, Nafees B, Narewska J, et al. Health state utilities for metastatic breast cancer. Br j cancer. 2006;95(6):683–690.
- Healthcare Pricing Office (HPO). ABF 2020 admitted patient price list: dRG prices for inpatients and daycases 2020. 2020.
- Thielen FW, van Dongen-Leunis A, Arons AMM, et al. Cost-effectiveness of anti-CD19 chimeric antigen receptor T-cell therapy in pediatric relapsed/refractory B-cell acute lymphoblastic leukemia. A societal view. Eur J Haematol. 2020;105(2):203–215.
- Ernst&Young LLP. Analysis of hospital activity and costs following allogeneic stem cell transplantation in England. London: 2021.
- Bourke S, Burns RM, Gaynor C. Challenges in generating costs and utilisation rates associated with castration-resistant prostate cancer. J Mark Access Health Policy. 2014;2(1):10.3402/jmahp.v2.24072.
- Ara R, Brazier JE. Populating an economic model with health state utility values: moving toward better practice. Value Health. 2010;13(5):509–518.
- Central Statistics Office (CSO). Consumer price index 2020. Available from: https://data.cso.ie/.
- Organisation for Economic Co-operation and Development (OECD). Purchasing power parities (PPP) 2020. Available from: https://data.oecd.org/conversion/purchasing-power-parities-ppp.htm.
- Tilly H, Gomes da Silva M, Vitolo U, et al. Diffuse large B-cell lymphoma (DLBCL): eSMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(Suppl 5):v116–25. DOI:10.1093/annonc/mdv304
- Ibrahim Y-A, Christian C, Peter B, et al. Management of adults and children undergoing chimeric antigen receptor T-cell therapy: best practice recommendations of the european society for blood and marrow transplantation (EBMT) and the joint accreditation committee of ISCT and EBMT (JACIE). Haematologica. 2020;105(2):297–316. DOI:10.3324/haematol.2019.229781
- Crump M, Kuruvilla J, Couban S, et al. Randomized comparison of gemcitabine, dexamethasone, and cisplatin versus dexamethasone, cytarabine, and cisplatin chemotherapy before autologous stem-cell transplantation for relapsed and refractory aggressive lymphomas: nCIC-CTG LY.12. J Clin Oncol. 2014;32(31):3490–3496. DOI:10.1200/JCO.2013.53.9593
- National Centre for Pharmacoeconomics (NCPE). About us. Health Technology Assessment. 2021 [cited 2022 Mar 03]. Available from: https://www.ncpe.ie/about/.
- McCullagh L, Barry M. The pharmacoeconomic evaluation process in Ireland. Pharmacoeconomics. 2016;34(12):1267–1276.
- Brennan A, Kharroubi S, O’Hagan A, et al. Calculating partial expected value of perfect information via monte carlo sampling algorithms. Med Decis Making. 2007;27(4):448–470.
- Wood S Mgcv: mixed GAM computation vehicle with automatic smoothness estimation. 2021 [cited 2021 Nov 05]. Available from: https://cran.r-project.org/web/packages/mgcv/index.html.
- Strong M. R code for partial EVPI section of public health, school of health and related research. University of Sheffield; [cited 2021 Sept 28]. Available from: https://www.sheffield.ac.uk/scharr/people/staff/mark-strong.
- National Institute for Health and Care Excellence (NICE). CHTE methods review: discounting - task and finish group report. 2020.
- Gravelle H, Smith D. Discounting for health effects in cost-benefit and cost-effectiveness analysis. Health Econ. 2001;10(7):587–599.
- Norwegian Medicines Agency (NoMA). Single technology assessment: axicabtagene ciloleucel (Yescarta) for the treatment of second or later relapsed/refractory diffuse large B-cell lymphoma (DLBCL) and primary mediastinal large B-cell lymphoma (PMBCL). 2018.
- Lin VW, Blaylock B, Epstein J, et al. Systematic literature review of health-related quality of life among aggressive non-Hodgkin lymphoma survivors. Curr Med Res Opin. 2018;34(8):1529–1535.
- Lin V, Jiang Y, Chuang L, et al. Health utilities for patients with relapsed or refractory large B-cell lymphoma (R/R-LBCL): ad hoc analysis from an axicabtagene ciloleucel (axi-cel) safety managenebt study. Bone Marrow Transplant. 2018;53:878–887.
- Qi CZ, Bollu V, Yang H, et al. Cost-effectiveness analysis of tisagenlecleucel for the treatment of patients with relapsed or refractory diffuse large B-cell lymphoma in the United States. Clin Ther. 2021;43(8):1300–1319.e8.
- Moradi-Lakeh M, Yaghoubi M, Seitz P, et al. Cost-effectiveness of tisagenlecleucel in paediatric acute lymphoblastic leukaemia (pALL) and adult diffuse large B-cell lymphoma (DLBCL) in Switzerland. Adv Ther. 2021;38(6):3427–3443.
- Rutherford SC, Leonard JP. Lymphoma “benchmark” or “bench-smudge”? Blood. 2017;130(16):1778–1779.
- Sermer D, Batlevi C, Palomba ML, et al. Outcomes in patients with DLBCL treated with commercial CAR T cells compared with alternate therapies. Blood Adv. 2020;4(19):4669–4678. DOI:10.1182/bloodadvances.2020002118
- Cher BP, Gan KY, Aziz MIA, et al. Cost utility analysis of tisagenlecleucel vs salvage chemotherapy in the treatment of relapsed/refractory diffuse large B-cell lymphoma from Singapore’s healthcare system perspective. J Med Econ. 2020;23(11):1321–1329. DOI:10.1080/13696998.2020.1808981
- Rahman R, Ventz S, McDunn J, et al. Leveraging external data in the design and analysis of clinical trials in neuro-oncology. Lancet Oncol. 2021;22(10):e456–65. DOI:10.1016/S1470-2045(21)00488-5
- Philips Z, Claxton K, Palmer S. The half-life of truth: what are appropriate time horizons for research decisions? Med Decis Making. 2008;28(3):287–299.
