ABSTRACT
Introduction: Primary immune thrombocytopenia is a rare autoimmune disease characterised by a decreased platelet count resulting in an increased risk of bleeding events and even life-threatening haemorrhages. Thrombopoietin receptor agonists (TPO-RAs) are the standard of care second-line therapy for adult patients with chronic immune thrombocytopenia. The first TPO-RAs approved and reimbursed in Italy, eltrombopag and romiplostim, while effective, pose some issues in terms of safety (e.g., hepatotoxicity) or general management (e.g., dietary restrictions). Avatrombopag, an effective and well-tolerated TPO-RA, was recently granted reimbursement.
Methods: A 3-year (2023–2025) budget impact analysis (BIA) was conducted to estimate its impact on the Italian National Health Service (NHS). Two scenarios were compared, of which one represents the current situation, without avatrombopag, and the other provides for an increasing market share of avatrombopag (up to 26.6%).
Results: BIA shows that the increase in the use of avatrombopag correlates with savings for NHS: in the first year, saving would be €1,300,564, increasing to €2,774,210 in the third year, for a total of €6,083,231 over the 3-year period. The sensitivity analysis confirmed these savings in the scenario with avatrombopag.
Conclusions: Based on this BIA, the introduction and reimbursement of avatrombopag is an efficient and advantageous choice for the Italian NHS.
Background and objectives
Immune thrombocytopenia (ITP) is a rare autoimmune disease characterised by a decreased platelet count associated with both accelerated platelet destruction and megakaryopoiesis impairment [Citation1]. It is referred to as ‘primary’ when it is not associated with other comorbidities or ‘secondary’ when an underlying medical condition (e.g., autoimmune diseases, viral infections, tumours) can be identified. The primary forms account for approximately 80% of ITPs, and the secondary forms for approximately 20% [Citation2]. In Italy, ITP has an estimated prevalence of 23.6 cases per 100,000 inhabitants [Citation3] and an incidence of 3.3 cases per 100,000 inhabitants [Citation4].
Three phases are in treatment international guidelines based on disease duration: the acute phase, from diagnosis to 3 months post-diagnosis, the persistent phase, from 3 to 12 months after diagnosis, and the chronic phase, where the disease persists beyond 12 months [Citation5–7].
Paediatric ITPs are usually secondary to infectious events and tend to self-limit, with only a minority of cases becoming chronic. Conversely, adult ITPs usually present without apparent triggering events and in most cases they tend to persist until the chronic phase of the disease [Citation8].
ITP, particularly in its chronic form, has a profound impact on patients’ quality of life, as a result of both the variable haemorrhagic manifestations and the associated chronic systemic symptoms, with consequent repercussions in various contexts (work, social life, sport, etc.) [Citation9].
The haemorrhagic manifestations most typical of ITP are mucocutaneous bleeding, followed by genitourinary and gastrointestinal bleeding. Major or fatal haemorrhagic manifestations are rare, although the risk can be increased by certain factors such as old age, the presence of multiple comorbidities or concomitant use of anti-platelet or anticoagulant medication [Citation10].
Although the platelet count cut-offs may not precisely reflect the bleeding risk of the individual patient, the risk of haemorrhage generally increases with a platelet count below 50,000/mm3 and becomes significant for values under 20–30,000/mm3. The bleeding risk is highest below 10,000 platelets/mm3 [Citation10].
In accordance with current international guidelines, the first-line therapies are [Citation6,Citation7]: a) corticosteroids, to which only 25% of patients respond (beyond six weeks of therapy) [Citation5,Citation11–13] and for which long-term treatment is associated with common side effects [Citation6,Citation14,Citation15]; b) IVIg (Intravenous Immunoglobulins) and/or anti-D immunoglobulins (Ig) that can be administered in intravenous infusions, which generate a transient response and are associated with adverse events [Citation5,Citation12,Citation13]. For adult patients with ITP who do not achieve remission or do not respond to first-line therapy with corticosteroids or immunoglobulins, current international guidelines indicate the need for second-line treatment [Citation6]. The main second-line therapies for ITP that are currently approved and reimbursed are thrombopoietin receptor agonists (TPO-RAs), which constitute the standard of care for the majority of patients [Citation7,Citation16,Citation17]. The other second-line drugs that are reimbursed in Italy include fostamatinib, only for patients who are refractory or have contraindications for at least one TPO-RA, and rituximab [Citation18]. Rituximab is used off-label and is reimbursed under Italian Law 648/96 [Citation19].
The first TPO-RAs to be approved and reimbursed in Italy were eltrombopag (ELT) and romiplostim (ROM), drugs that, despite being effective, are associated with some issues [Citation20–22]. ELT, while requires dietary restrictions, is associated with alanine transaminase and bilirubin elevation, for which it has a Boxed Warning regarding a risk of severe and life-threatening hepatotoxicity, and requires caution and close monitoring, especially in patients with liver disease [Citation23]. ROM is associated with the development/progression of the formation of reticulin fibres in the bone marrow and possible bone marrow fibrosis, as well as with injection site reactions being subcutaneously administered [Citation24–26].
The latest TPO-RA reimbursed in Italy, avatrombopag (AVA), can be taken orally without food-type restrictions [Citation27–29]. Treatment with AVA has been shown to result in rapid and long-lasting achievement of a platelet count above 50,000/mm3 after just eight days of treatment in two out of three patients. This increase occurs gradually from 3–5 days after the start of treatment, and reaches peak effect after 10–13 days [Citation28,Citation30]. Patients treated with AVA generally maintained a platelet count of between 50,000/mm3 and 150,000/mm3 for the duration of the pivotal Phase 3 study. AVA is well-tolerated, has demonstrated that it is not associated with significant hepatotoxicity, and is characterised by a low incidence of thromboembolic events [Citation28,Citation30]. Pooling the patients with ITP in 4 clinical studies, thromboembolic events were observed in 9/128 patients [Citation29]; furthermore, AVA has been associated with a reduction in the use of concomitant therapies for the management of ITP [Citation28,Citation30].
Based on these results, AVA was granted reimbursement for the treatment of chronic primary ITP in adult patients who are refractory to other treatments (such as corticosteroids and immunoglobulins) [Citation31]. In order to evaluate the economic/financial impact on the Italian National Health Service (NHS), following the reimbursement of AVA, a budget impact analysis (BIA) over a time horizon of 3 years was carried out.
Materials and methods
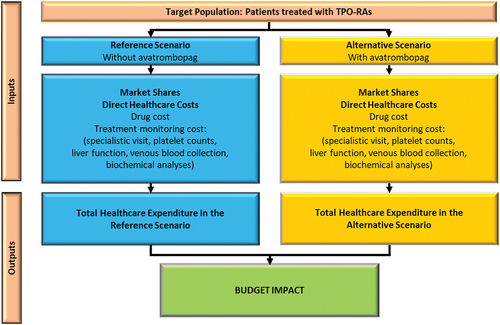
The BIA () was conducted in compliance with the Guidelines issued by both the International Society for Health Economics and Outcomes Research (ISPOR) and the Italian Medicines Agency (AIFA) [Citation32,Citation33]. All the hypotheses used in the analysis were validated by two Italian clinical experts and one health economic expert. The analysis was developed from the perspective of the Italian NHS over a time horizon of three years (2023–2025).
The number of patients with chronic primary ITP was estimated based on literature sources, by applying the chronic primary ITP prevalence, incidence and growth rates for the entire adult (≥18 years) Italian population for the period considered [Citation3,Citation4,Citation34]. This population was further analysed in relation to the strategy applied and the corresponding health outcomes to identify the population potentially eligible for treatment with TPO-RAs (target population). It was assumed that 80% of patients with ITP have a primary form and that 71% of the latter require first-line treatment [Citation35,Citation36].
Only a portion of these patients is actually treated with TPO-RAs. In the BIA, these patients were identified as follows:
Prevalent patients (which received a diagnosis at least two year before the present study and are currently treated), were considered to already have a chronic form, with 55.2% of them requiring a line of treatment subsequent to the first [Citation35]. Lastly, using market data it was calculated that 38.3% of the subjects identified above are already treated with a TPO-RA [Citation37].
For incident patients (i.e., those diagnosed with the disease in a year the model refers to), it was estimated that 80% of patients evolve towards the chronic form [Citation38] and that 55.2% require a line of treatment subsequent to the first [Citation35]. Lastly, in accordance with the opinion of the clinical experts, it was estimated that in clinical practice approximately 80% of these patients are treated with a TPO-RA.
The epidemiological estimates are shown in . In the analysis, the population is considered to remain constant and stable over the three years of the time horizon.
Table 1. Target population*†.
Two scenarios are compared in the BIA: a Reference Scenario, without AVA, and an Alternative Scenario, involving the progressive introduction of AVA into the current TPO-RA market, to the detriment of the two reimbursed drugs: ELT and ROM. The values are shown in .
Table 2. Estimated patients and market shares broken down by year and therapy: base case and sensitivity analysis *†.
For the economic quantification, the data obtained from the literature were confirmed and integrated by means of a specific questionnaire administered to the clinical experts to identify the actual patient journey and resource consumption in relation to the therapies used, in terms of average dose of the drug and average annual frequency of specialist consultations and diagnostic tests and procedures.
Consultations and diagnostic, follow-up and laboratory tests were valorised using the outpatient fees currently applicable in Italy [Citation39]. The frequencies and costs associated with monitoring are shown in . More specifically, in the Base Case an average AVA dose of 17.7 mg/day was used (pooled analysis of data from phase 2/3 trials) [Citation40], whereas for ELT the mean dose of the EXTEND study (51.3 mg/day) was used [Citation41,Citation42]. For ROM, on the other hand, given the extremely variable doses, the analysis used the weighted mean of the packs sold in Italy as a function of the respective market shares [Citation37]: 67.6% for the 250 mcg pack and 32.4% for the 500 mcg pack (329.75 mcg per week). For conservative reasons, 100% compliance was assumed for all the drugs.
Table 3. Monitoring costs per patient.
The drug therapy costs for AVA, ELT and ROM were estimated considering the ex-factory prices minus the mandatory manufacturer discounts (−5%, −5%) [Citation43]. (), assuming an absence of drug wastage and an average weight of 70 kg (in accordance with AIFA Guidelines) [Citation33]. The analysis does not consider administration costs, since AVA and ELT are oral drugs, whereas ROM is administered subcutaneously.
Table 4. Cost per patient/year: base case and sensitivity analysis.
In order to assess the robustness of the Base Case results, the authors carried out a deterministic one-way sensitivity analysis, in which the dose used was varied and, more specifically, the doses stated in the technical data sheet were used for AVA (20 mg/day) and ELT (50 mg/day), whereas for ROM, in agreement with the clinical experts, an average weekly dose of 5 mcg per kg of body weight (350 mcg per week) was used [Citation23,Citation29]. It was decided to vary only the dose of the drug, since this is the main cost driver.
Results
Target population
The number of adult patients with chronic primary ITP treated with or potentially eligible for treatment with TPO-RAs was estimated to be constant and equal to 1,741 patients in the three years of the analysis. Of these, the number of patients treated with AVA in the Alternative Scenario was 212 in the first year (TPO-RA-naïve) and 463 in the third year (of whom 137 TPO-RA-naïve). The values are shown in .
Patient journey: monitoring consultations and tests
The results of the questionnaire showed that the frequency and type of tests and consultation varied depending on the therapy used:
AVA = 16 consultations in the first year and 12 consultations from the second year onwards, for venous blood draws and platelet counts
ELT = 16 consultations in the first year and 12 consultations from the second year onwards, for venous blood draws, platelet counts and liver function tests (ALT, AST and total and fractionated bilirubin)
ROM = 20 consultations in the first year and 12 consultations from the second year onwards, for venous blood draws and platelet counts
These results, which were used in both the Base Case and the Sensitivity Analysis, showed: a higher number of consultations and tests in the first year (for all treatments) compared to subsequent years; a higher number of consultations and tests in the first year for ROM compared to other therapies; need for a liver function tests for ELT alone vs other therapies ().
Base case results
In the Base Case, as indicated in , the per-patient annual drug therapy cost was estimated to be: AVA = € 26558.36; ELT = € 29029.80; ROM = € 37295.25. These costs were constant over the three years of the model.
On the contrary, monitoring costs varied depending on the year of treatment of the patients.
The data show a lower cost for AVA, since for the first year of ROM therapy consultations and tests are more frequent, and for ELT the costs associated with liver function tests must be considered. It therefore follows that the average annual per-patient cost differs depending on the treatment strategy used, linked with the costs for monitoring.
Overall, AVA proves to be the least costly drug for the NHS:
AVA = €27,157 (of which € 598.71 for monitoring) for the first year and € 26875 (€ 316.92 for monitoring) for the subsequent years.
ELT = € 29680 (€ 650.46) for the first year and € 29338 (€ 358.32) for the subsequent years.
ROM = € 38000 (€ 704.35) for the first year and € 37612 (€ 316.92) for the subsequent years.
The overall results of the model are presented as the differential between the reference scenario, which does not provide for the inclusion of AVA as an available treatment option, and the alternative scenario, which provides for an incremental increase of the use of AVA in the same overall number of patients treated over the three years analysed. The drivers considered include the drug therapy costs and the costs of the services generated by the various monitoring regimens considered. These results show, against three-year expenditure with AVA of € 27032,626 (16.6% of total expenditure), cost saving for the NHS of € 1,300,564 in the first year, of € 2,008,457 in the second and of € 2,774,210 in the third, for a total saving over the three-year period of € 6,083,231 (, ).
Figure 2. Budget Impact and healthcare expenditure variation per year, linked with avatrombopag reimbursement (Alternative – Reference scenario) per year: base case analysis.
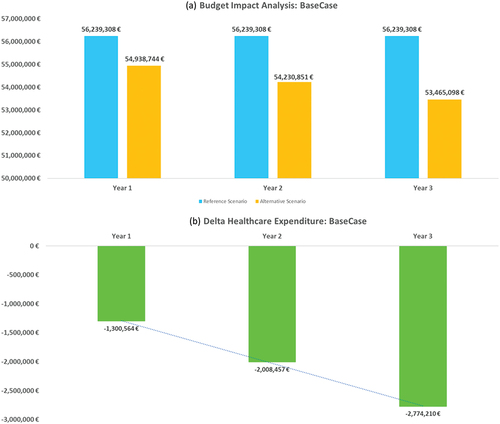
Table 5. Budget impact analysis: base case analysis.*
Sensitivity analysis
In this scenario, the doses of therapy were modified in accordance with those stated in the technical data sheet, since the cost of drug therapy was seen to be the most important driver in the total healthcare costs estimate, keeping the monitoring costs stable for conservative reasons.
In the Sensitivity Analysis () the estimated per-patient annual cost is:
AVA = € 30608 (of which € 598.71 for monitoring) for the first year and € 30326 (€ 316.92 for monitoring) for the subsequent years.
ELT = € 28945 (€ 650.46) for the first year and € 28652 (€ 358.32) for the subsequent years.
ROM = € 40290 (€ 704.35) for the first year and € 39903 (€ 316.92) for the subsequent years.
In the sensitivity analysis, ELT was the drug with the lowest healthcare costs and was due to the lower dose of ELT (50 mg/day) used in this setting [Citation23].
In the Sensitivity Analysis (, ), the overall expenditure for the three-year period (drug therapy and monitoring) is € 170,358,975 in the Reference Scenario vs € 167,129,125 in the Alternative Scenario. The comparison between the scenarios shows that against total expenditure (drug therapy and monitoring) for AVA of € 30487,165 (18.2% of total expenditure) the cost savings would be € 3,229,850. Although it presents hypotheses that are less favourable for AVA, the Sensitivity Analysis confirms the robustness of the model and the estimates.
Figure 3. Budget Impact and healthcare expenditure variation per year, linked with avatrombopag reimbursement (Alternative – Reference scenario) per year: sensitivity analysis.
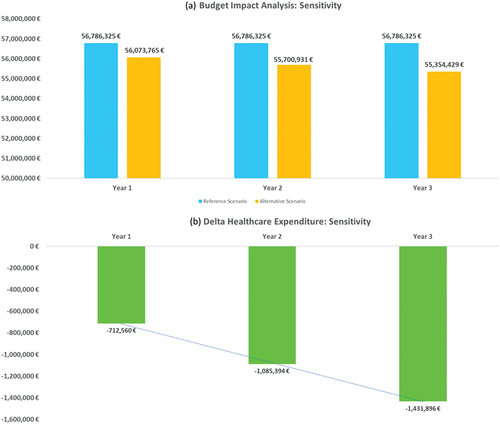
Table 6. Budget impact analysis: sensitivity analysis.*
Discussion
The BIA was developed to estimate the impact on healthcare costs (drug therapy and monitoring) as a function of the reimbursement and increased use of AVA in Italy.
It was developed by adopting a conservative approach to represent the possible cost of the therapies depending on the doses and the monitoring regimen. The data showed that the doses are different to those of the technical data sheets, with a lower consumption of AVA and a lower consumption of ELT [Citation23,Citation29,Citation40–42]. As romiplostim schedule followed a personalized approach on patient’s weight basis, consumption data coming from market research and the opinion of Italian clinical experts are considered proxies of the dosage in the clinical practice, in line with AIFA Guidelines for Economic Evaluations [Citation33,Citation37]. The data therefore demonstrate the variability of the results as a function of the doses, illustrating that the cost of therapy is the major driver of this analysis. Although AVA has lower monitoring costs (same-year comparison) than the comparators, an assessment of the results shows an increase in expenditure for monitoring between the first and second years as a function of the higher number of patients being treated with a new TPO-RA. This is because all TPO-RAs have higher monitoring costs in the first year of treatment and in the model AVA gains markets shares from both naïve patients and those already treated with the other TPO-RAs. As a matter of fact, for all patients starting therapy with AVA it is assumed that the same first-year tests and consultations will be repeated, regardless of the previous therapies.
To compare the results of the analysis with other international and national papers, an ad hoc literature search was carried out, focusing on the studies published over the last 10 years. Unfortunately, there are no other studies that have investigated the economic and financial implications of AVA in this indication, making it impossible to compare our results with other studies. On the other hand, the studies comparing ELT and ROM yielded conflicting results, but tend to favour ELT. The study by Allen et al. analysed the cost of treatment in England and Wales, reaching the conclusion that therapy with ELT is less expensive than that with ROM, with better results in non-splenectomised patients than in splenectomised subjects [Citation44]. The studies by Tremblay et al. and Patwardhan et al., both referring to costs in the United States, reached similar conclusions, with a lower cost for ELT [Citation45,Citation46]. On the contrary, the study by Fust et al., in which the authors developed a cost-effectiveness analysis, calculating the incremental cost effectiveness ratio (ICER) as a function of the incremental number of responder patients, concluded that ROM is more cost-effective than ELT, given its greater effectiveness and lower costs associated. The conclusions can therefore differ depending on the type of analysis presented [Citation47].
The first limitation of this analysis is the variability of the results depending on the doses used and, therefore, the costs of therapy. It was therefore decided to perform the Sensitivity Analysis, modifying the doses of the therapies. However, it should be pointed out that the Base Case data appear to be particularly robust [Citation37,Citation40,Citation41] and that they should represent real practice better than just using the doses stated on the technical data sheet [Citation23,Citation24,Citation29]. Furthermore, in both analyses, ROM would appear to be the most expensive drug, whereas the introduction of AVA always coincides with a reduction in the overall three-year expenditure for the NHS.
The second limitation of the analysis is the absence of adverse event assessments. They were not included because AVA was studied vs placebo and because in most cases the adverse events were mild or moderate, including: headache (29.8%), bruising (40.4%) and upper respiratory tract infections (23.4%). These frequencies were similar to those observed in the placebo group [Citation27]. Furthermore, it should also be noted that the cost of drug therapy is the major driver of the analysis and that it is unlikely that including the costs related to adverse events would have a considerable impact on the results presented.
Conclusions
Based on this budget impact analysis the introduction and reimbursement of AVA in the treatment of ITP, in adult patients who are refractory to other treatments, is an efficient and advantageous choice for the Italian NHS, with savings in terms of both pharmaceutical expenditure and the expenditure associated with monitoring the therapies. The robustness of the analysis was confirmed also by the sensitivity analysis which has considered very conservative and challenging assumptions for AVA.
Disclosure statement
AA, EEM, and MP are employees of Intexo Società Benefit S.r.l. that received funding from Sobi, Milan, Italy for this research; NM and CT are employees of Sobi, Milan, Italy; SC, GC and SS report consultancy fees from Sobi, Milan, Italy, for contributing to this research. Financial support: Sobi, Milan, Italy, provided financial support to cover the cost of this project.
References
- Cooper N, Ghanima W Immune Thrombocytopenia. Solomon CG. editor N Engl J Med. Internet 2019 Sep 5;381(10):945–11. doi: 10.1056/NEJMcp1810479
- Cines DB, Bussel JB, Liebman HA, et al. The ITP syndrome: pathogenic and clinical diversity. Blood Internet 2009 Jun 25;113(26):6511–6521. doi: 10.1182/blood-2009-01-129155
- Feudjo-Tepie MA, Robinson NJ, Bennett D. Prevalence of diagnosed chronic immune thrombocytopenic purpura in the US: analysis of a large US claim database: a rebuttal [5]. J Thromb Haemost. 2008;6(4):711–712. doi: 10.1111/j.1538-7836.2008.02911.x
- Rodeghiero F, Marranconi E. Management of immune thrombocytopenia in women: current standards and special considerations. Expert Rev Hematol. Internet 2020;13(2):175–185. doi: 10.1080/17474086.2020.1711729
- Rodeghiero F, Stasi R, Gernsheimer T, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood Internet 2009 Mar 12;113(11):2386–2393. https://ashpublications.org/blood/article/113/11/2386/109971/Standardization-of-terminology-definitions-and
- Neunert C, Terrell DR, Arnold DM, et al. American society of hematology 2019 guidelines for immune thrombocytopenia. Blood Adv. 2019;3(23):3829–3866. doi: 10.1182/bloodadvances.2019000966
- Provan D, Arnold DM, Bussel JB, et al. Updated international consensus report on the investigation and management of primary immune thrombocytopenia. Blood Adv. 2019;3(22):3780–3817. doi: 10.1182/bloodadvances.2019000812
- Despotovic JM, Grimes AB. Pediatric ITP: is it different from adult ITP? Hematology. Internet 2018 Nov 30;2018(1):405–411. doi: 10.1182/asheducation-2018.1.405
- Cooper N, Kruse A, Kruse C, et al. Immune thrombocytopenia (ITP) World Impact Survey (I-WISh): impact of ITP on health-related quality of life. Am J Hematol. Internet 2020 Feb 19;96(2):199–207. doi: 10.1002/ajh.26036
- Arnold DM. Bleeding complications in immune thrombocytopenia. Hematology. 2015 Dec 5;2015(1):237–242. Internet doi: 10.1182/asheducation-2015.1.237
- Matzdorff A, Meyer O, Ostermann H, et al. Immune thrombocytopenia - current diagnostics and therapy: recommendations of a joint working group of DGHO, ÖGHO, SGH, GPOH, and DGTI. Oncol Res Treat. 2018;41(Suppl 5):1–30. doi: 10.1159/000492187
- Provan D, Newland AC. Current management of primary immune thrombocytopenia. Adv Ther. 2015;32(10):875–887. doi: 10.1007/s12325-015-0251-z
- Neunert CE. Management of newly diagnosed immune thrombocytopenia: can we change outcomes? Blood Adv. 2017;1(24):2295–2301. doi: 10.1182/bloodadvances.2017009860
- Berti D, Moons P, Dobbels F, et al. Impact of corticosteroid-related symptoms in patients with immune thrombocytopenic purpura: results of a survey of 985 patients. Clin Ther. 2008;30(8):1540–1552. doi: 10.1016/j.clinthera.2008.08.005
- Brown TM, Horblyuk RV, Grotzinger KM, et al. Patient-reported treatment burden of chronic immune thrombocytopenia therapies. BMC Blood Disord. Internet 2012;12(1):2. doi: 10.1186/1471-2326-12-2
- Neunert C, Noroozi N, Norman G, et al. Severe bleeding events in adults and children with primary immune thrombocytopenia: a systematic review. J Thromb Haemost. Internet 2015 Mar;13(3):457–464. https://linkinghub.elsevier.com/retrieve/pii/S1538783622086627
- An R, Wang PP. Length of stay, hospitalization cost, and in-hospital mortality in US adult inpatients with immune thrombocytopenic purpura, 2006–2012. Vasc Health Risk Manag. 2017;13:15–21. doi: 10.2147/VHRM.S123631
- Agenzia Italiana del Farmaco. Determina n. 1060/2021. Available from: https://www.gazzettaufficiale.it/atto/serie_generale/caricaDettaglioAtto/originario?atto.dataPubblicazioneGazzetta=2021-09-21&atto.codiceRedazionale=21A05458&elenco30giorni=true.
- Agenzia Italiana del Farmaco. Legge 648/96. Lista faramaci ad uso consolidato. Available from: https://www.aifa.gov.it/legge-648-96
- Williams DD, Peng B, Bailey CK, et al. Effects of food and antacids on the pharmacokinetics of eltrombopag in healthy adult subjects: two single-dose, open-label, randomized-sequence, crossover studies. Clin Ther. 2009 Apr;31(4):764–776. https://linkinghub.elsevier.com/retrieve/pii/S0149291809001192
- Gilbert MM, Grimes AB, Kim TO, et al. Romiplostim for the treatment of immune thrombocytopenia: spotlight on patient acceptability and ease of use. Patient Prefer Adherence. 2020;14: 1237–1250. doi: 10.2147/PPA.S192481
- Bussel JB, Kuter DJ, Pullarkat V, et al. Safety and efficacy of long-term treatment with romiplostim in thrombocytopenic patients with chronic ITP. Blood. Internet 2009;113(10):2161–2171. doi: 10.1182/blood-2008-04-150078
- European Medicine Agency. REVOLADE. Summary of product characteristics. Available from: https://www.ema.europa.eu/en/documents/product-information/revolade-epar-product-information_en.pdf.
- European Medicine Agency. NPLATE. Summary of product characteristics. Available from: https://www.ema.europa.eu/en/documents/product-information/nplate-epar-product-information_en.pdf.
- Ghanima W, Junker P, Hasselbalch HC, et al. Fibroproliferative activity in patients with immune thrombocytopenia (ITP) treated with thrombopoietic agents. Br J Haematol. 2011;155(2):248–255. doi: 10.1111/j.1365-2141.2011.08845.x
- Kuter DJ, Mufti GJ, Bain BJ, et al. Evaluation of bone marrow reticulin formation in chronic immune thrombocytopenia patients treated with romiplostim. Blood. Internet 2009 Oct 29;114(18):3748–3756. https://ashpublications.org/blood/article/114/18/3748/26572/Evaluation-of-bone-marrow-reticulin-formation-in
- Jurczak W, Chojnowski K, Mayer J, et al. Phase 3 randomised study of avatrombopag, a novel thrombopoietin receptor agonist for the treatment of chronic immune thrombocytopenia. Br J Haematol. 2018;183(3):479–490. doi: 10.1111/bjh.15573
- European Medicine Agency. DOPTELET. European assessment report. Available from: https://www.ema.europa.eu/en/documents/variation-report/doptelet-h-c-004722-ii-0004-g-epar-assessment-report-variation_en.pdf
- European Medicine Agency. DOPTELET. Summary of product characteristics. Available from: https://www.ema.europa.eu/en/documents/product-information/doptelet-epar-product-information_en.pdf
- Poordad F, Terrault NA, Alkhouri N, et al. Avatrombopag, an alternate treatment option to reduce platelet transfusions in patients with thrombocytopenia and chronic liver disease-integrated analyses of 2 phase 3 studies. Int J Hepatol. 2020;2020:1–11. doi: 10.1155/2020/5421632
- Agenzia Italiana del Farmaco. Determina n. 327/2022. Available from. https://www.gazzettaufficiale.it/atto/serie_generale/caricaDettaglioAtto/originario?atto.dataPubblicazioneGazzetta=2022-05-23&atto.codiceRedazionale=22A03007&elenco30giorni=true
- Sullivan SD, Mauskopf JA, Augustovski F, et al. Budget impact analysis—Principles of good practice: report of the ISPOR 2012 budget impact analysis good practice II task force. Value Heal. Internet 2014 Jan;17(1):5–14. https://linkinghub.elsevier.com/retrieve/pii/S1098301513042356
- Agenzia Italiana del Farmaco. Linee guida per la compilazione del dossier a supporto della domanda di rimborsabilità e prezzo. Available from: https://www.aifa.gov.it/documents/20142/1283800/Linee_guida_dossier_domanda_rimborsabilita.pdf
- Istituto Nazionale di Statistica (ISTAT). Previsioni della Popolazione Anni 2011-2065. Available from:http://demo.istat.it/. 2012. p. 22–25.
- Palandri F, Polverelli N, Sollazzo D, et al. Have splenectomy rate and main outcomes of ITP changed after the introduction of new treatments? A monocentric study in the outpatient setting during 35 years. Am J Hematol. 2016;91(4):E267–72. doi: 10.1002/ajh.24310
- Lambert MP, Gernsheimer TB. Clinical updates in adult immune thrombocytopenia. Blood. 2017;129(21):2829–2835. doi: 10.1182/blood-2017-03-754119
- IQVIA. Market research. Data on file.
- Kistangari G, McCrae KR. Immune thrombocytopenia. Hematol Oncol Clin North Am. 2013;27(3):495–520. doi: 10.1016/j.hoc.2013.03.001
- Ministero S. Decreto 18/10/2012 Remunerazione prestazioni di assistenza ospedaliera per acuti, assistenza ospedaliera di riabilitazione e di lungodegenza post acuzie e di assistenza specialistica ambulatoriale.
- Bussel J, Allen LF, Aggarwal K, et al. Lack of clinically significant hepatotoxicity in patients with chronic immune thrombocytopenia (c-ITP) treated with the novel, oral thrombopoietin receptor agonist avatrombopag-poole.
- Wong RSM, Saleh MN, Khelif A, et al. Safety and efficacy of long-term treatment of chronic/persistent ITP with eltrombopag: final results of the EXTEND study. Blood. Internet 2017;130(23):2527–2536. doi: 10.1182/blood-2017-04-748707
- The National Institute for Health and Care Excellence. Eltrombopag for treating chronic immune (idiopathic) thrombocytopenic purpura - Technology appraisal guidance [Internet]. 2014 Jul. Available from: www.nice.org.uk/guidance/ta293
- Patient Access Monitor. Prezzi dei farmaci. Available from: https://pamonitor.it/LoginNew.aspx?ReturnUrl=%2fFarmaList.aspx
- Allen R, Bryden P, Grotzinger KM, et al. Cost-effectiveness of eltrombopag versus romiplostim for the treatment of chronic immune thrombocytopenia in England and wales. Value Heal. Internet 2016;19(5):614–622. doi: 10.1016/j.jval.2016.03.1856
- Tremblay G, Dolph M, Bhor M, et al. Cost-consequence model comparing eltrombopag versus romiplostim for adult patients with chronic immune thrombocytopenia. Clin Outcomes Res. Internet 2018;10:705–713.Nov. doi: 10.2147/CEOR.S177324
- Patwardhan P, Proudman D, Allen J, et al. Cost-minimization analysis comparing eltrombopag vs romiplostim for adults with chronic immune thrombocytopenia. J Manag Care Spec Pharm. 2021;27(10):1447–1456. doi: 10.18553/jmcp.2021.21080
- Fust K, Parthan A, Li X, et al. Cost per response analysis of strategies for chronic immune thrombocytopenia. Am J Manag Care. 2018;24:SP294–302.