ABSTRACT
Background: The benefits of preventive interventions lack comprehensive evaluation in standard health technology assessments (HTA), particularly for rare and transmissible diseases.
Objective: To identify possible considerations for future HTA using analogies between the treatment and prevention of rare diseases.
Study design: An Expert panel meeting assessed whether one HTA assessment framework can be applied to assess both rare disease treatments and preventive interventions. Experts also evaluated the range of value elements currently included in HTAs and their applicability to rare, transmissible, and/or preventable diseases.
Results: A broad range of value should be considered when assessing rare, transmissible disease prevention. Although standard HTA can be applied to transmissible diseases, the risk of local outbreaks and the need for large-scale prevention programs suggest a modified assessment framework, capable of incorporating prevention-specific value elements in HTAs. A ‘Rule of Prevention’ framework was proposed to allow broader value considerations anchored to severity, equity, and prevention benefits in decision-making for preventive interventions for rare transmissible diseases.
Conclusion: The proposed prevention framework introduces an explicit initial approach to consistently assess rare transmissible diseases, and to incorporate the broader value of preventive interventions compared with treatment.
Introduction
Established health technology assessment (HTA) bodies tend to focus on clinical and cost-effectiveness, largely with a health-payer or health system focus. Cost-effectiveness analysis (CEA) is often applied to assess health benefits of interventions (i.e., vaccines, medical devices, or pharmaceutical drugs) using quality-adjusted life-years (QALY), and cost-savings. The health system perspective often adopted by HTA bodies potentially excludes other comprehensive health, societal and economic benefits an intervention may offer (e.g., value of hope, family spillovers, reducing fear of contagion and disease, out-of-pocket expenses and social care costs) [Citation1]. Decision makers rely on consistent and comparable approaches to ensure a degree of equity in the distribution of healthcare resources. These approaches, however, may inadequately assess the benefit derived from interventions which may deliver alternative sources of value [Citation1,Citation2], and special considerations such as societal preferences for more equitable access to healthcare or better health equity may not always be captured [Citation2–4].
Currently, HTA appraisals allow flexible considerations for special cases in which standardized approaches either cannot capture the full value of an intervention or where society holds outcomes in greater value, notably severity of disease effect, genetic predisposition, disproportionate impact on younger cohorts, innovation, and scale of unmet need [Citation5]. The moral compulsion to rescue patients from imminent death, regardless of the cost, was first described as the ‘Rule of rescue’ by Albert Jonsen [Citation6,Citation7]. Decision-makers, however, face challenges to balance the affordability of healthcare for the many with the ethical arguments to save the few in dire need.
Therapies for rare and very rare diseases are often treated as special cases in HTA, given their low prevalence, disease severity, high unmet need, and limited treatment options potentially driving an urgency to access therapeutic alternatives. However, small patient numbers, limited follow-up, and high patient heterogeneity combined with (often) high treatment costs may not demonstrate cost-effectiveness under the standard appraisal processes. Some HTAs recognize the need to amend the standard approaches, including weighting QALYs, accepting higher cost-effectiveness thresholds, exemption from economic evaluation, or separate appraisal pathways (e.g., giving patient and other stakeholder perspectives a stronger role in determining value) [Citation8,Citation9]. Even with HTA adaptations for special cases, defining the unmet need differs by geography, intervention, and treatment availability. There is a lack of transparency, consistency, and clarity regarding decision-making, and the absence of more definitive criteria or an evaluation framework makes generalization across all interventions difficult.
The rare disease or orphan drug framework was established to support developments for diseases affecting small populations, such as genetic disorders (which account for 80% of rare diseases [Citation10]) or rare cancers. In the case of rare transmissible diseases, the size of the affected population may increase temporarily if infections result in an outbreak. The disease can, therefore, be classified as common, within a limited geography/population at a specific time, but also classed as rare, due to the infrequency of outbreaks and their limited geographic concentration. Notably, for interventions preventing rare transmissible diseases (i.e., vaccination or alternative interventions), the flexible or special HTA considerations often do not apply, and there is less clarity on an appropriate framework for evaluation. This is because target populations for vaccination can be larger than other interventions, putting vaccination at a disadvantage relative to other health and non-health interventions. illustrates these distinctions of rare/non-rare disease types, transmissible/non-transmissible nature of diseases, prevention/treatment options, and the potential overlap between these characteristics, which can influence HTA considerations. These added complexities may result in different considerations when assessing prevention of rare transmissible diseases versus treatment of rare diseases.
Figure 1. Illustration of disease and intervention characteristics, and overlaps, which can impact HTA considerations.
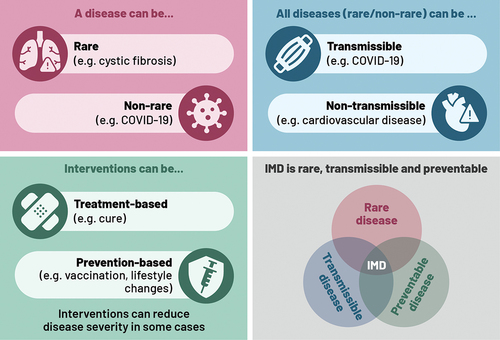
For instance, invasive meningococcal disease (IMD) is a rare, severe, and transmissible disease that can be life-threatening in the acute phase with prolonged hospitalization, as well as a risk of long-term disability in over 40% of survivors [Citation11]. Incidence is highest in infants and young children followed by adolescents and places a health and financial burden on the family and caregivers [Citation12,Citation13]. IMD has a rapid onset and progression, with a high risk of mortality, and it is often not identified in time for effective antibiotic treatment. Therefore, prevention of IMD through vaccination is the most effective option. Special HTA considerations (i.e., spillover effects, indirect costs, and societal preferences) were needed for the vaccine approval in the United Kingdom [Citation14] (considerations which many geographies may not adopt or consider within their HTA).
An expert panel convened to explore analogies between the assessment of interventions to treat rare diseases and vaccines to prevent rare transmissible diseases (using IMD as an example). Our aim was to understand how to further develop and assist HTA (applicable to vaccines and other interventions) for rare transmissible diseases, i.e., to allow evaluation of their full value, in an equitable manner, and using an appropriate framework.
Material and methods
Eleven experts participated in an Expert panel meeting in January 2021, from the European Union (EU), United Kingdom (UK), and the United States (US). Experts were selected for their knowledge and publications on country-specific healthcare policy and HTA practices as well as clinical knowledge of infectious diseases (i.e., IMD) and/or health economics and orphan drug expertise. Prior to the meeting, each expert was given pre-read materials, completed a survey on key HTA questions (around analogies between rare and rare transmissible diseases to determine similarities and differences), and aligned on objectives and context of the meeting.
The first phase of the Expert panel meeting was organized to explore:
How rare diseases are defined and valued and the orphan drug criteria that are acceptable for HTA approval
HTA modifiers or special (ethical) evaluations that can be applied to rare transmissible diseases
How IMD and IMD vaccination fit within a rare disease framework.
The key similarities and differences, and the implications of these for HTA were determined i.e., can IMD vaccination be assessed with the same broader considerations used for orphan drugs?
The second phase of the Expert panel meeting was to understand the:
Differences in assessments of preventive interventions, vaccines, and rare diseases
Value of prevention and its current recognition in healthcare and HTA
Value of prevention and health equity considerations
The benefits of interventions for rare diseases, transmissible diseases, and preventable diseases, and how they are currently captured in HTA/CEA.
Regarding the terminology, a rare disease (e.g., cystic fibrosis) is defined as a disease affecting fewer than 1 in 2,000 people in the US and EU [Citation10]. Transmissible diseases (e.g., COVID-19, influenza) are infectious diseases which can be transmitted from an infected person to others. A disease can be both rare and transmissible (e.g., Ebola). Many transmissible diseases can be prevented through vaccination. Preventable diseases also include non-transmissible diseases (e.g., type 2 diabetes, cardiovascular disease), which can be prevented through lifestyle changes and medication (see ).
Expert panel discussion led to a consensus that a ‘Rule of Prevention’ (analogous to the rule of rescue) could be warranted to explore the benefits of preventive interventions in rare transmissible diseases. Following the meeting, each expert was contacted individually to provide further reflection, independent interpretation, and a summary of the Expert panel discussion. Also, a selected expert group reviewed the range of potential intervention benefits (using published value frameworks) and their applicability to rare, transmissible, or preventable non-transmissible diseases. The group also began to conceptualize an initial framework for the ‘Rule of Prevention’ to support possible future developments in HTA or provide a basis for decision-making for preventive interventions.
Results
The results first present 1) similarities and differences between rare versus rare transmissible diseases (e.g., IMD), and between orphan drugs versus preventive interventions; 2) key decision-making factors to consider when assessing preventive interventions for rare transmissible diseases; 3) benefits (value elements) of interventions for rare diseases, transmissible diseases, and non-transmissible preventable diseases and their inclusion in HTA; 4) the proposed Rule of Prevention framework; and 5) a case study applying the framework to IMD.
Comparing rare diseases and rare transmissible diseases
Experts agreed that IMD resembles a rare disease based on the criteria presented in (e.g., disease burden, unmet need, and low incidence criteria similar to a rare disease [Citation15]), except during local outbreaks, when case numbers increase temporarily. However, IMD differs because disease transmission is unpredictable and, with the risk of outbreaks, potentially on a larger scale.
Figure 2. HTA valuation in rare diseases and application to IMD shows the key characteristics of rare diseases and how orphan drugs are assessed by HTA on the left, and the similarities and differences when applied to invasive meningococcal disease (IMD) and vaccination, on the right.Abbreviations: HIV: human immunodeficiency virus; HTA: health technology assessment; IMD: invasive meningococcal disease; QoL: quality of life
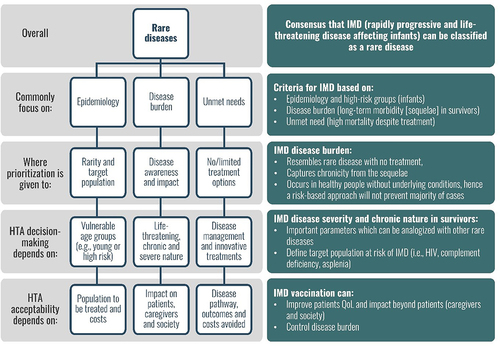
While IMD may be comparable to a rare disease, IMD vaccination would be challenged under the orphan drug criteria, as the target population differs, i.e., vaccination targets a large group of healthy people to prevent disease, whereas orphan drugs target a small group of very sick people to treat the disease (contributing to differences in public fund allocation). The transmissible nature of IMD and the preventive nature of the intervention could imply that current broad HTA considerations for orphan drugs may not be applicable to IMD vaccination.
Prevention, severity, and equity – key considerations in decision-making
Prevention benefits may be less visible than treatment benefits
Society implicitly values prevention highly, both within and outside of healthcare and can influence some policies from routine infant vaccination (e.g., through public petitions) [Citation16] to transport infrastructure interventions designed to reduce accidents [Citation17]. Compared with treatments for highly visible severe diseases, however, disease prevention tends to be a lower priority in the health sector, and especially prevention of rare diseases. Despite the availability of funded prevention programs, the use of preventive services remains low (e.g., 8% of US adults used recommended preventive services for chronic conditions [Citation18] and adult vaccination rates remain well below targets [Citation19]). While HCPs are aware of these preventive options, implementation problems [Citation18], a lack of HCP recommendation for vaccination [Citation19], and a focus on urgent problems which can be treated rather than prevention of future health issues play a role [Citation20]. The UK government estimated that spending on prevention was around 5% of the health service’s annual budget, while the rest was spent on treatment, despite greater health returns from prevention. There is now a greater focus on investing in prevention, to achieve health targets [Citation21].
Preventive interventions (e.g., vaccination) and treatment are different. The coronavirus disease 2019 (COVID-19) pandemic (although not a rare disease but one with severe or long-term consequences) has illustrated the far-reaching benefits of vaccination (during an epidemic) and the critical role of prevention in maintaining public health and supporting healthcare systems. The excess value of prevention versus treatment has also been highlighted [Citation22,Citation23]. Prevention has excess unrecognized value over cure or treatment, i.e., with prevention, the individual does not get the disease; with cure, the individual has a period of ill-health but returns (at least in part) to full health – although long-term sequelae may persist; and with treatment, the individual lives in ill-health with diminished capacity. Key differentiating features of prevention, therefore, include the de facto value from preventing illness from ever occurring, its potential to eradicate or significantly reduce the incidence of a disease, and vaccination impact beyond vaccinated individuals (e.g., possibly protecting a larger group than just those vaccinated, and reducing fear of contagion).
Impact of severity is more important than impact of rarity for decision-making
The impact of disease severity was deemed more important for decision makers by the Expert panel than the implications of rarity and should be formally and consistently accounted for in future vaccine HTA/CEA. This is supported by a recent systematic review showing a societal preference for investing in rare diseases, which was driven by the disease severity, lack of alternative options, and a desire to be able to treat all people [Citation24]. Recently, the UK’s HTA guidelines, from the National Institute for Health and Care Excellence (NICE), have been updated to allow greater weight in CEA for health benefits in severe diseases (replacing greater weight for end-of-life health gains), as well as allowing more flexibility with regard to uncertainty when generation of supporting evidence is challenging, such as for rare diseases [Citation25,Citation26]. Although NICE does not apply the ‘Rule of rescue’ explicitly, its Highly Specialized Technologies program (appraising ultra-orphan drugs for very rare and severe diseases and allowing for greater flexibility with regard to cost-effectiveness) may well result in decisions that are in keeping with the rule [Citation6]. The Netherlands and Norway also explicitly account for disease severity in HTA quantitatively, with higher cost-effectiveness thresholds allowed for more severe diseases, assessed using absolute and proportional shortfall theory. Other countries, such as the US, account for severity implicitly, in a deliberative process [Citation27].
National prevention programs contribute to improving health equity
For transmissible diseases, national immunization programs (NIP) and regular screening/diagnostics have the potential to improve health equity where treatment may not, e.g., by preventing disease in sociodemographic groups with limited or poor access to treatment (low health-seeking behavior) and in groups at risk for transmissible diseases (e.g., due to lifestyle/living conditions affected by health inequality strata, such as socioeconomic status) [Citation28].
In the US, as part of the Evidence to Recommendations framework to assess the value of vaccines and inform Advisory Committee on Immunization Practices (ACIP) decisions, a new domain of importance to decision makers was added: to assess the impact of (COVID-19) vaccines on health equity (i.e., impact in potential disadvantaged groups and social determinants that reduce access to healthcare) [Citation29].
Value elements deemed important to HTA/CEA
There has been extensive research into how to broaden the value perspective considered in HTA, and presents a range of existing and proposed value elements [Citation1,Citation30]. The experts assessed which elements are currently assessed, and which are relevant for future assessment of rare diseases, transmissible diseases, and non-transmissible preventable diseases. Many important factors for rare, transmissible, or preventable diseases are not commonly or explicitly considered in the assessment phases of HTA (). Firstly, there are challenges in collecting data for rare diseases, which may be even more challenging for rare transmissible diseases, due to the nature and heterogeneity of outbreaks. Secondly, collecting data for some of the new proposed value elements may be challenging, due to limited evidence or a lack of validated scales (e.g., to quantify the value of hope or scientific spillovers) [Citation31]. However, although the population level impact of contagion and fear of contagion can be difficult to quantify, some survey methods have already been proposed as a solution, to determine the willingness-to-pay (WTP) to avoid exposure [Citation30]. Other value elements may only be partially considered in current HTA, e.g., productivity linked to affected individuals (or caregivers) is included in the US and the Netherlands [Citation31]; however, the impact of transmissible diseases on workplaces and the wider economy is generally not considered [Citation1,Citation30].
Table 1. Value elements (based on [1, 30]), and experts’ assessment of their inclusion in HTA/CEA, and their applicability to rare, transmissible, and non-transmissible preventable diseases. presents a range of published value elements are assesses which ones are currently included in Health Technology Assessment (HTA)/cost-effectiveness analysis (CEA), and which applicable to rare diseases, transmissible diseases and non-transmissible preventable diseases.
Inclusion of a broad range of value elements in HTA/CEA frameworks is likely to introduce uncertainty surrounding the supporting evidence available, given the current lack of data or quantification methods for some value elements. Thirdly, challenges exist relating to the valuation of prevention versus treatment, especially prevention of rare diseases, e.g., there is residual and variable uncertainty regarding the consequences of failing to prevent disease, including impacts on long-term individual health, increases in antimicrobial resistance, reductions in hospital capacity to treat illnesses, and others [Citation32,Citation33].
Developing a Rule of Prevention in rare and severe, transmissible preventable diseases
The ‘Rule of rescue’ focusses on the immediate need to save patients already suffering from severe and life-threatening disease and, thus, does not apply to preventive interventions such as vaccination, administered in healthy populations. As the value of prevention is less well represented, the experts proposed that a ‘Rule of Prevention’ framework should be developed with prevention, severity, and equity as core values, to allow these benefits to be accounted for more consistently in HTA, when no curative treatment options exist.
As current HTA/CEA does not consider the broader benefits of interventions (including vaccination), e.g., peace of mind and financial security to the individual from preventing severe disease and long-term complications, the true value is likely to be higher than currently estimated. However, an allowance should be made for greater uncertainty in benefits accrued from broader value elements. Based on these two principles, a prevention framework () could allow for a broader value assessment of interventions that are often underserved by conventional HTA (e.g., for rare and severe, transmissible, preventable diseases), with considerations for potential willingness to accept broader value elements and greater related uncertainty. The approach proposed has value for severe preventable diseases which are rare or non-rare, transmissible or non-transmissible.
Figure 3. Key considerations when applying the Rule of prevention in HTA – disease severity impact and acceptance of broader benefits and uncertainty. shows the Rule of Prevention framework developed, and highlights the key considerations for its use i.e., disease severity impact and acceptance of broader benefits and uncertainty.Abbreviations: CEA: cost-effectiveness analysis; HTA: health technology assessment; WTP: willingness-to-pay
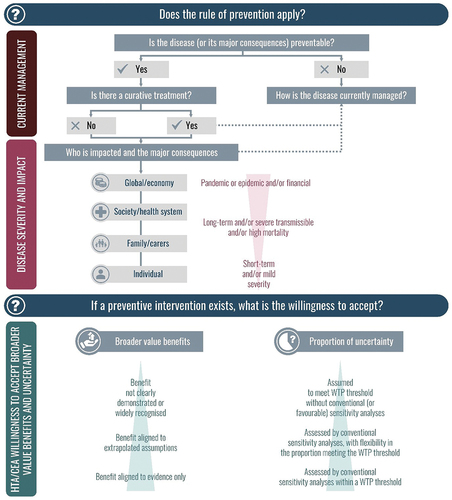
Just as HTA uses special considerations in restricted cases [Citation27], the ‘Rule of Prevention’ should be considered when there is a preventive intervention and no curative treatment, and, with greater flexibility to allow for broader value benefits, despite uncertainty, in more severe transmissible diseases with important consequences beyond the patient alone ().
Willingness to accept a broader range of value elements reflects an understanding that a broader perspective is required to account for all hidden or under-recognized value elements of prevention, i.e., moving from narrow benefits to broad societal benefits in the HTA analysis [Citation34]. A broader range of prevention value benefits for HTA consideration has recently been established [Citation35], including concepts such as reducing institutional disruptions, political stability, health systems strengthening, and preventing loss of leisure time. The Rule of Prevention framework provides a transparent way to include additional broader value elements (from the many proposed in recent research) along with consideration of their uncertainty. The additional value elements included in the assessment will depend on the disease and intervention considered.
Accounting for excess uncertainty with the addition of broader value benefits can be complex, involving parameter uncertainty due to additional value benefits, or structural uncertainty related to the unknown long-term consequences of preventive interventions. For example, herd immunity benefits cannot be assessed in clinical trials, but are estimated from real-world evidence with its associated uncertainty. More flexibility may be required when interpreting cost-effectiveness outcomes, e.g., variable WTP thresholds, and greater acceptance of uncertainty from (probabilistic and deterministic) sensitivity analyses ().
Case study: Application of the Rule of Prevention to IMD
IMD survivors may experience a range of physical/neurological sequelae (including amputation, blindness, deafness, and skin scarring), and psychological/behavioral sequelae which are often under-reported [Citation12] and can lead to the need for special education [Citation36] (i.e., manifesting 36 months after diagnosis or later, and including anxiety, behavioral and depressive disorders) [Citation11]. Families/caregivers are also affected in the acute phase and due to permanent disabilities in patients, as caring for the patient can lead to spillover effects including anxiety and depression [Citation37]. The wider societal burden includes productivity losses in patients and in caregivers [Citation38], the potential for outbreaks, dependence on government financial benefits [Citation39], and a public health management burden [Citation40].
IMD and its associated sequelae can be prevented by vaccination, and there are no curative alternatives – IMD vaccination is therefore a candidate for the Rule of prevention framework.
Including broader value elements in CEA improved the cost-effectiveness of IMD vaccination, i.e., impact of long-term sequelae, impact on caregivers and family, economic impact beyond the healthcare system, societal preferences to give more weight to prevention of very severe diseases and, special economic considerations for discount rates to account for long-term consequences of IMD [Citation41]. In a recent analysis using distributional CEA methods to assess equity impact, IMD vaccination was found to improve health equity which increased the cost-effectiveness of the vaccination program [Citation42]. Some of these factors were associated with greater uncertainty due to lack of data and relied on assumptions and sensitivity analyses [Citation41]. In the case of IMD epidemiology, uncertainty remains regarding the occurrence and impact of outbreaks [Citation43]. Some additional broad benefits (e.g., reducing fear of contagion, insurance value, and value of hope) that cannot be included/integrated in CEA (due to lack of methods or evidence) may be considered in the appraisal part of the HTA.
IMD vaccination, particularly for serogroup B IMD in the UK [Citation44], has benefitted from special HTA considerations, and various recommendations were put forward for future evaluations, e.g.,, to consider indirect benefits, QALY adjustment factors, and variable WTP thresholds [Citation41,Citation45], to assess vaccines within the total government budget instead of ringfenced healthcare budget [Citation40], or to use alternative economic methods such as extended cost-effectiveness analysis, value of statistical life measures, value of risk reduction and cost–benefit analyses [Citation46].
Discussion
Research on appraising preventive interventions demands a societal perspective to include broader prevention-specific benefits, e.g., indirect benefits and macroeconomic gains [Citation35]. HTA bodies recognize that special considerations are needed when standard approaches cannot capture the full value of an intervention and to reflect societal preferences, e.g., to prioritize severe diseases, greater unmet needs, impact on younger cohorts, or to reward innovation [Citation47,Citation48]. HTA bodies may be flexible regarding cost-effectiveness [Citation7], accept added benefit, or offer alternative appraisal pathways [Citation49,Citation50]. Where there is HTA decision uncertainty, special funds and managed access agreements can allow restricted patient access to interventions, while additional evidence is collected, e.g., the UK Cancer Drugs Fund and Innovative Medicines Fund [Citation51] and innovation funds in some European countries [Citation52]. Typically, broader value is considered for severe diseases or to prevent imminent death, following ‘Rule of rescue’ principles [Citation53].
The ‘Rule of Prevention’ framework allows the inclusion of much broader prevention benefits, including beyond the healthcare system, and the possibility of benchmarking against other and non-health prevention programs. A common comparative measure could be the implicit value of a statistical life, using return on investment tools [Citation54]. For example, the cost per statistical life was €10 million for a water project in the Netherlands compared with €30k for the influenza vaccination program [Citation54]. Appropriate information systems need to be developed to collect the range of data needed (and make use of data already available) for a broader value analysis and to reduce uncertainty about long-term indirect benefits of disease prevention. The acceptability of including macroeconomic data [Citation31] should be assessed.
The assessment of vaccines differs from therapeutics, involving variable country-specific value assessments by the National Immunization Technical Advisory Groups (NITAG), sometimes followed by HTA reimbursement decisions [Citation55]. A recent review of value elements included in assessments has highlighted a lack of systematic consideration of many broad benefits specific to prevention [Citation31]. The ‘Rule of Prevention’ framework should be flexible to account for factors such as disease severity, societal preferences, uncertainty in the data and outcomes, and different risk-benefit ratios, influenced by disease incidence. Multi-criteria decision analysis methods [Citation2] used for orphan drugs could be applied, where dimensions relevant to the disease and intervention are defined and then weighted, e.g., prioritizing severity and health-related quality of life impact. The use of weights, or ‘modifiers’ [Citation25], and the role and value of a variable ‘sliding’ WTP threshold [Citation27] should be considered.
Recently, several new frameworks have emerged to address specific methods and value concepts that are currently lacking in vaccine HTA/CEA. In addition to the value elements presented in , they introduce the value of prevention in supporting health systems (e.g., freeing up hospital resources and capacity by preventing disease, to allow further investments and other diseases to be treated) and the critical value of preventing diseases which require antimicrobial treatment and thus potentially promote antimicrobial resistance [Citation35]. Aimed at vaccination and vaccine-preventable disease in general, these frameworks are also applicable to rare transmissible disease and tend to propose expanding CEA by broadening the range of value elements included in the analysis, to better reflect the true cost-effectiveness of vaccination programs [Citation31]. Common to these approaches, however, is to provide decision makers a broader perspective of the value of prevention to allow for more accurate value assessments and more equitable patient access.
Existing HTA bodies are often limited in how they capture the wider benefits of preventive interventions and employ broader considerations to assess rare diseases, or other special cases. The value of prevention is not adequately or consistently captured in HTA currently; therefore, a ‘Rule of Prevention’ framework was put forward to better assess severe preventable diseases (although should not be limited to this framework). The framework argues for inclusion and acceptance of broader prevention benefits, such as societal and macroeconomic benefits, while acknowledging the need for flexibility in addressing the greater uncertainty this will bring. More research, development and understanding are needed on the applicability, operationalization, and overall acceptability of such a framework within HTA, to aid decision-making around the value of prevention within and beyond rare transmissible disease.
Disclosure statement
Daniel Molnar, Kinga Meszaros, Woo-Yun Sohn are employed by and hold shares in GSK. Najida Begum is a freelancer c/o GSK. All other authors received funding from GSK for the initial expert panel participation and not for the development of this manuscript. Federico Martinón-Torres reports other from Ablynx, grants and other from Jansen, personal fees, non-financial support and other from GSK, other from Regeneron, other from Medimmune, personal fees, non-financial support and other from Pfizer, grants, personal fees, non-financial support and other from MSD, personal fees and other from Sanofi Pasteur, personal fees and other from Novavax, grants from Astra Zeneca, other from Novartis, personal fees, non-financial support and other from Seqirus, other from Roche, other from Abbott, other from Instituto de Salud Carlos III , personal fees from Biofabri, outside the submitted work. David E. Bloom has received grant funding from the National Institute of Aging, the Carnegie Corporation of New York, the UNFPA, the Bill and Melinda Gates Foundation, Sanofi, Telethon Kids Institute, and the International Vaccine Initiative. He has also served on advisory boards, received speaking fees and travel reimbursements, taught in Executive Education programs, and worked on studies relevant to health technology assessment. These activities have been funded by Pfizer, Sanofi, GSK, Merck, Simpson Healthcare, the Davos Alzheimer’s Collaborative, the Bill and Melinda Gates Foundation, the International Vaccine Initiative, and the Welcome Trust. David Salisbury reports consultation work with GSK, Pfizer, Sanofi, AstraZeneca, Seqirus, Merck and Clover Pharmaceuticals. Federico JL Severens reports grants and other from Alexion, AstraZeneca, Ferrer, Gilead, GSK, Kite, Nefemed/Diagned, and SHE Collaborates, and consultancy fees and others from Alira Health, Axentiva, Boston Consultancy Group, IQVIA, Luman Value & Access, Lumanity, Maple Health Group, Mtech Access, and Access Infinity. Daniel Ollendorf reports grants and other from Merck Sharp & Dohme, Genentech, the Commonwealth Fund, PhRMA Foundation, and Arnold Ventures. The authors declare no other financial and non-financial relationships and activities.
Additional information
Funding
References
- Lakdawalla DN, Doshi JA, Garrison LP, et al. Defining elements of value in health care—a health economics approach: an ISPOR special task force report [3]. Value Health. 2018;21(2):131–11. doi: 10.1016/j.jval.2017.12.007
- Angelis A, Kanavos P, Montibeller G. Resource allocation and priority setting in health care: a multi-criteria decision analysis problem of value? Global Policy. 2017;8(S2):76–83. doi: 10.1111/1758-5899.12387
- Gustavsson E, Tinghög G. Needs and cost-effectiveness in health care priority setting. Health Technol. 2020;10(3):611–619. doi: 10.1007/s12553-020-00424-7
- World Health Organization (WHO). Strategizing national health in the 21st century: a handbook - Priority-setting for national health policies, strategies and plans (Chapter 4) 2016 [Cited: 2022 June 16]. Available from: https://apps.who.int/iris/bitstream/handle/10665/250221/9789241549745-chapter4-eng.pdf;jsessionid=AA42AFA70BCBE0D49F850FA2DC5F7311?sequence=36.
- Reckers-Droog V, van Exel J, Brouwer W. Equity weights for priority setting in healthcare: severity, age, or both? Value Health. 2019;22(12):1441–1449. doi: 10.1016/j.jval.2019.07.012
- Charlton V. Does NICE apply the rule of rescue in its approach to highly specialised technologies? J Med Ethics. 2022 Feb;48(2):118–125. doi: 10.1136/medethics-2020-106759
- Cookson R, McCabe C, Tsuchiya A. Public healthcare resource allocation and the rule of rescue. J Med Ethics. 2008;34(7):540–544. doi: 10.1136/jme.2007.021790
- Blonda A, Denier Y, Huys I, et al. how to value orphan drugs? A review of European value assessment frameworks. Front Pharmacol. 2021;12:631527. doi: 10.3389/fphar.2021.631527
- Nicod E, Whittal A, Drummond M, et al. Are supplemental appraisal/reimbursement processes needed for rare disease treatments? An international comparison of country approaches. Orphanet J Rare Dis. 2020 Jul 20;15(1):189. doi: 10.1186/s13023-020-01462-0
- Frederiksen SD, Avramović V, Maroilley T, et al. Rare disorders have many faces: in silico characterization of rare disorder spectrum. Orphanet J Rare Dis. 2022 Feb 22;17(1):76.
- Shen J, Begum N, Ruiz-Garcia Y, et al. Range of invasive meningococcal disease sequelae and health economic application - a systematic and clinical review. BMC Public Health. 2022 May 31;22(1):1078. doi: 10.1186/s12889-022-13342-2
- Guedes S, Bricout H, Langevin E, et al. Epidemiology of invasive meningococcal disease and sequelae in the United Kingdom during the period 2008 to 2017 - a secondary database analysis. BMC Public Health. 2022;22(1). doi: 10.1186/s12889-022-12933-3
- Davis K, Misurski D, Miller J, et al. Cost impact of complications in meningococcal disease: evidence from a United States managed care population. Hum Vaccines. 2011;7(4):458–465. doi: 10.4161/hv.7.4.14434
- Christensen H, Trotter CL, Hickman M, et al. Re-evaluating cost effectiveness of universal meningitis vaccination (Bexsero) in England: modelling study. BMJ. 2014 Oct 9;349:5725. doi: 10.1136/bmj.g5725
- National Organization for Rare Disorders (NORD). Meningococcal meningitis [Cited: 2022 June 21]. Available from: https://rarediseases.org/rare-diseases/meningococcal-meningitis/.
- Royal College of Nursing. Immunisation: Royal College of Nursing; 2021 [Cited: 2022 June 21]. Available from: https://www.rcn.org.uk/clinical-topics/public-health/immunisation.
- Public Health England (PHE). Reducing unintentional injuries on the roads among children and young people under 25 years 2018 [Cited: 2022 June 21]. Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/695781/Reducing_unintentional_injuries_on_the_roads_among_children_and_young_people_.pdf.
- Levine S, Malone E, Lekiachvili A, et al. Health care industry insights: Why the use of preventive services is still low. Prev Chronic Dis. 2019;16. doi: 10.5888/pcd16.180625.
- Centers for Disease Control and Prevention (CDC). Majority of US adults are missing routine vaccinations 2021 [ Cited: 01-06-2023]. Available from: https://www.cdc.gov/vaccines/hcp/adults/for-practice/increasing-vacc-rates.html.
- SocialFinance.org. Why don’t we fund more prevention? 2019 [ Cited: 01-06-2023]. Available from: https://socialfinance.org/insight/why-dont-we-fund-more-prevention/.
- Stephen M, James L, Karl C. Is an ounce of prevention worth a pound of cure? A cross-sectional study of the impact of English public health grant on mortality and morbidity. BMJ Open. 2020;10(10):e036411. doi: 10.1136/bmjopen-2019-036411
- Talic S, Shah S, Wild H, et al. Effectiveness of public health measures in reducing the incidence of covid-19, SARS-CoV-2 transmission, and COVID-19 mortality: systematic review and meta-analysi. BMJ. 2021;375(68302):e068302. doi: 10.1136/bmj-2021-068302
- Mykhalovskiy E, French M. COVID-19, public health, and the politics of prevention. Sociology Of Health And Illness. 2020;42(8):4–15. doi: 10.1111/1467-9566.13192
- Dabbous O, Chachoua L, Aballéa S, et al. Valuation of treatments for rare diseases: a systematic literature review of societal preference studies. Adv Ther. 2023 Feb;40(2):393–424.
- National Institute for Health and Care Excellence (NICE). Changes we’re making to health technology evaluation 2022 [Cited: 2022 June 21]. Available from: https://www.nice.org.uk/about/what-we-do/our-programmes/nice-guidance/nice-technology-appraisal-guidance/changes-to-health-technology-evaluation.
- National Institute for Health and Care Excellence (NICE). Centre for health technology evaluation (CHTE) methods review - Developing the manual, Task and finish group report 2021 [Cited: 2022 April 22]. Available from: https://www.nice.org.uk/Media/Default/About/what-we-do/our-programmes/nice-guidance/chte-methods-and-processes-consultation/developing-the-manual-tfg-report.docx.
- Skedgel C, Henderson N, Towse A, et al. Considering severity in health technology assessment: can we do better? Value Health. 2022 Aug 01;25(8):1399–1403.
- Taha MK, Martinon-Torres F, Köllges R, et al. Equity in vaccination policies to overcome social deprivation as a risk factor for invasive meningococcal disease. Expert Rev Vaccines. 2022 Mar;29:1–16.
- Oliver S ACIP COVID-19 vaccines. EtR framework: Public health problem, resource use and equity domains 2020 [Cited: 2022 April 22]. Available from: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2020-11/COVID-02-Oliver.pdf.
- Jit M, Hutubessy R, En Png M, et al. The broader economic impact of vaccination: reviewing and appraising the strength of evidence. BMC Med. 2015;13(209):1–9. doi: 10.1186/s12916-015-0446-9
- Postma M, Biundo E, Chicoye A, et al. Capturing the value of vaccination within health technology assessment and health economics: Country analysis and priority value concepts. Vaccine. 2022 Jun 26;40(30):3999–4007. doi: 10.1016/j.vaccine.2022.04.026
- Bonanni P, Picazo JJ, Rémy V. The intangible benefits of vaccination – what is the true economic value of vaccination? J Mark Access Health Policy. 2015;3:3. doi: 10.3402/jmahp.v3.26964
- Doherty MT, Aris E, Servotte N, et al. Capturing the value of vaccination: impact of vaccine-preventable disease on hospitalization. Aging Clin Exp Res. 2022 July 01;34(7):1551–1561.
- Bärnighausen T, Bloom DE, Cafiero ET, et al. Economic evaluation of vaccination: capturing the full benefits, with an application to human papillomavirus. Clin Microbiol Infect. 2012 Oct;18(5):70–76.
- Beck E, Biundo E, Devlin N, et al. Capturing the value of vaccination within health technology assessment and health economics: Literature review and novel conceptual framework. Vaccine. 2022 Jun 26;40(30):4008–4016. doi: 10.1016/j.vaccine.2022.04.050
- Sumpter R, Brunklaus M, McWilliam R, et al. Health-related quality-of-life and behavioural outcome in survivors of childhood meningitis. Brain Inj. 2011;25(13–14):1288–1295. doi: 10.3109/02699052.2011.613090
- Al-Janabi H, Van Exel J, Brouwer W, et al. Measuring Health Spillovers for Economic Evaluation: A Case Study in Meningitis. Health Econ. 2016;25(12):1529–1544. doi: 10.1002/hec.3259
- Olbrich K, Muller D, Schumacher S, et al. Systematic review of invasive meningococcal disease: sequelae and quality of life impact on patients and their caregivers. Infect Dis Ther. 2018;7(4):421–438. doi: 10.1007/s40121-018-0213-2
- Shen J, Bouée S, Aris E, et al. Long-term mortality and state financial support in invasive meningococcal disease-real-world data analysis using the French national claims database (SNIIRAM). Infect Dis Ther. 2022 Feb;11(1):249–262.
- Sevilla J, Tortorice D, Kantor D, et al. editors. Lifecycle model-based economic evaluation of infant meningitis B vaccination in the UK. Copenhagen: ISPOR; 2019.
- Beck E, Klint J, Neine M, et al. Cost-effectiveness of 4CMenB infant vaccination in England: a comprehensive valuation considering the broad impact of serogroup b invasive meningococcal disease. Value Health. 2021;24(1):91–104. doi: 10.1016/j.jval.2020.09.004
- Dronova M, Biundo E, Chicoye A, et al. editors. The value of vaccination: capturing the impact of vaccination on health equity in health economic analysis. Vienna Austria: ISPOR Europe; 2022.
- Martinón-Torres F, Trilla A. Meningococcal disease: Can we predict the unpredictable? Med Clin. 2020 Jan 10;154(1):20–22. doi: 10.1016/j.medcli.2019.04.035
- Christensen H, Trotter CL, Hickman M, et al. Re-evaluating cost effectiveness of universal meningitis vaccination (Bexsero) in England: modelling study. BMJ. 2014 Oct 9;349(oct09 4):g5725. doi: 10.1136/bmj.g5725
- Christensen H, Al-Janabi H, Levy P, et al. Economic evaluation of meningococcal vaccines: considerations for the future. Eur J Health Econ. 2020;21(2):297–309. doi: 10.1007/s10198-019-01129-z
- Mauskopf J, Masaquel C, Huang L. Evaluating vaccination programs that prevent diseases with potentially catastrophic health outcomes: how can we capture the value of risk reduction? Value Health. 2021;24(1):86–90. doi: 10.1016/j.jval.2020.06.018
- Linley WG, Hughes DA. Societal views on NICE, cancer drugs fund and value-based pricing criteria for prioritising medicines: a cross-sectional survey of 4118 adults in Great Britain. Health Econ. 2013;22(8):948–964. doi: 10.1002/hec.2872
- Ollendorf DA, Chapman RH, Pearson SD. Evaluating and valuing drugs for rare diseases: no easy answers. Value Health. 2018;21:547–557. doi: 10.1016/j.jval.2018.01.008
- Drummond MF, Wilson DA, Kanavos P, et al. Assessing the economic challenges posed by orphan drugs. Int J Technol Assess Health Care. 2007;23(1):36–42. doi: 10.1017/S0266462307051550
- Nicod E, Anneman L, Bucsics A, et al. HTA programme response to the challenges of dealing with orphan medicinal products: Process evaluation in selected European countries. Health Policy. 2019;123(2):140–151. doi: 10.1016/j.healthpol.2017.03.009
- NHS. NHS UK 2021 [Cited: 2022 June 6]. Available from: https://www.england.nhs.uk/2021/07/nhs-england-announces-new-innovative-medicines-fund-to-fast-track-promising-new-drugs/.
- Mayor S. New “managed access” process for cancer drugs fund to go ahead, NHS England confirms. BMJ. 2016;352. doi: 10.1136/bmj.i1208
- Jonsen AR. Bentham in a box: technology assessment and health care allocation. L Med Health Care. 1986;14(3–4):172–174. doi: 10.1111/j.1748-720X.1986.tb00974.x
- Goebbels AF, Ament AJ, Novák A, et al. Estimating the implicit value of statistical life based on public interventions implemented in the Netherlands. Int J Technol Assess Health Care. 2008 Fall;24(4):495–501.doi: 10.1017/S0266462308080653.
- Laigle V, Postma MJ, Pavlovic M, et al. Vaccine market access pathways in the EU27 and the United Kingdom -analysis and recommendations for improvements. Vaccine. 2021 Sep 15;39(39):5706–5718. doi: 10.1016/j.vaccine.2021.07.040
