ABSTRACT
Introduction: Only a selected proportion of chronic obstructive pulmonary disease (COPD) patients are managed in secondary care. The aim of this study was to characterize disease severity, treatment and structure of secondary care for COPD in Sweden.
Methods: Information was collected from 29 of 33 existing secondary care units of respiratory medicine in Sweden, using both individual data from 373 consecutively enrolled COPD patients with Global initiative on Obstructive Lung Disease (GOLD) stage III–IV and a structural questionnaire about available resources at the units. Patient data included exacerbations, health status assessed by COPD Assessment Test (CAT), lung function, comorbid conditions, pharmacological treatment and vaccinations. Structural data included available smoking cessation support, multidisciplinary rehabilitation, physical training, patient education and routine follow-up after exacerbations at the respective unit. All patients were reclassified according to the GOLD 2014 group A–D classification. Multiple linear regression investigated associations of available resources with number of exacerbations and CAT score.
Results: According to GOLD 2014, 87% of the population were GOLD D and 13% were GOLD C. Triple inhaled therapy were prescribed in 88% of the patients. Over 75% of the units had resources for smoking cessation, multidisciplinary rehabilitation, physical training and patient education. Routine follow-up after exacerbations was available in 35% of the units. Being managed at units with access to structured patient education was associated with statistically significantly fewer exacerbations (adjusted regression coefficient (95% confidence interval) −0.79 (−1.39 to −0.19), p = 0.010).
Conclusion: Most stage III–IV COPD patients managed at secondary care respiratory units in Sweden have maximized inhaled therapy and high risk disease even when reclassified according to GOLD 2014. Most units have access to smoking cessation, rehabilitation and patient education. Patients managed at units with structured patient education have a lower exacerbation risk.
Introduction
The prevalence of chronic obstructive pulmonary disease (COPD) in the adult population in Sweden is approximately 10%,[Citation1–Citation3] which is in accordance with global reports.[Citation4] The majority of Swedish COPD patients are handled in primary care, but as previously reported the most severe and resource utilizing patients are found in secondary care.[Citation5] However, the management of COPD in secondary care has previously not been surveyed in detail.
The major components of COPD management are smoking cessation, pharmacological treatment and pulmonary rehabilitation. All COPD patients should be correctly classified for disease severity and future risk of exacerbations, and receive optimized therapy according to current recommendations. Previously, Swedish national recommendations of treatment were based on the spirometric classification stage I to IV from Global initiative on Obstructive Lung Disease (GOLD) 2011, but since 2015 the updated guidelines of the National Board of Health and Welfare [Citation6] and the Medical Product Agency [Citation7] recommend a treatment schedule based on the recent GOLD COPD risk evaluation groups A–D, including lung function, symptoms and exacerbation frequency.[Citation8] It is also of utter importance that COPD care can offer support for smoking cessation, multidisciplinary rehabilitation including physical training, structured patient education and follow-up, in particular after exacerbations.[Citation6–Citation8]
The aim of the current study was to characterize the disease severity, treatment and structure of secondary respiratory care for COPD in Sweden.
Methods
Data collection
In Sweden, there are 33 hospital-based secondary care units for respiratory medicine, including departments of respiratory medicine or sections of respiratory medicine in departments of internal medicine. All 33 units were invited to participate in the present study. Data collection included both information from a structural questionnaire to the physician responsible for COPD care, and information from patient visits. Each respiratory unit was asked to consecutively enroll a maximum of 10 patients with GOLD grade III COPD and five patients with GOLD grade IV COPD [Citation8] during the period from 12 May 2011 to 28 March 2012. The proportions of stage III and IV patients were chosen to approximately match the distribution of severe and very severe COPD as assessed in the general population.[Citation9,Citation10] During the patients’ visits, information was collected by the physician on patient demographics, treatment, comorbidities, exacerbations, and health status. The information was entered into the study case record form together with data from the most recently performed spirometry. Post-bronchodilator values were used, but replaced with pre-bronchodilator values if post-bronchodilator values were missing (43%). The only exclusion criterion was an inability to complete the study on language grounds. The analyses of health status, comorbidities and frequent exacerbations have been described and published elsewhere.[Citation11,Citation12]
Variables
Patient data used in this study included sex, age, smoking history and habits, forced expiratory volume in one second in percentage of predicted value (FEV1% pred), body weight and height, number of exacerbations including hospitalizations due to exacerbations, health status using COPD assessment test (CAT), comorbidity, current pharmacological treatment and influenza and pneumococcal vaccination status.
An exacerbation was defined as worsening of symptoms of dyspnea and sputa beyond normal day to day variation.[Citation13] Frequent exacerbations were defined as having two or more exacerbations or one or more hospitalizations due to COPD exacerbations in the previous 12 months.[Citation8] The patients were classified into the following three groups according to BMI: BMI < 22, 22 ≤ BMI < 30 and BMI ≥ 30. The cut off-values were based on the prognostic importance of BMI in COPD reported in previous studies.[Citation14] Symptoms were categorized as less (CAT < 10) or more (CAT ≥ 10).[Citation8] Comorbid conditions were defined as conditions requiring medical treatment, and included cardiovascular disease, diabetes, renal dysfunction, musculoskeletal symptoms, osteoporosis and depression. COPD treatment groups included: long-acting muscarinic antagonists, long-acting beta-2-agonists, combined bronchodilators, combined long-acting beta-2-agonists and inhaled corticosteroids, triple inhaled therapy with long-acting muscarinic antagonists, long-acting beta-2-agonists and inhaled corticosteroids, long term oxygen therapy (LTOT) and roflumilast. Influenza vaccination referred to the previous year and pneumococcal vaccination to the previous five years.
Data from the structural questionnaires included information on the specific COPD guidelines that were used for disease management, and on access to smoking cessation support, multidisciplinary rehabilitation, physical training, patient education and planned follow-up after exacerbations, at the respective respiratory unit.
Statistics
Statistical analyses were performed using IBM Statistics SPSS version 22.0 (SPSS Inc., Chicago, IL, USA). The proportion of patients with different characteristics and pharmacological treatments were calculated. All patients were classified as GOLD 2011 stage III or IV, and reclassified as GOLD groups C or D according to GOLD 2014. Cross-tabulations and chi-squared test explored the association between patient characteristics with group C or D, and treatments with group C or D. Proportion of participating units with available COPD education, multidisciplinary rehabilitation, physical training, smoking cessation and planned follow-up at the own unit after exacerbations were calculated, as well as percentages of units using different treatment recommendations. Associations between available resources and total number of exacerbations and CAT score were analyzed by means of linear regression. The multiple model included sex, age and patients’ characteristics with statistically significant associations in the univariate model. In all analyses, a p-value below 0.05 was considered as statistically significant.
Ethics
The study was conducted as a non-interventional trial, in accordance with EU directive 2001/20/EC and the Declaration of Helsinki. The study protocol and the study was reviewed and approved by the Regional Ethical Review Board of Umeå University (Dnr 2011-10-31M). Oral and written informed consent was given by all patients.
Results
Classification of disease severity and patient characteristics
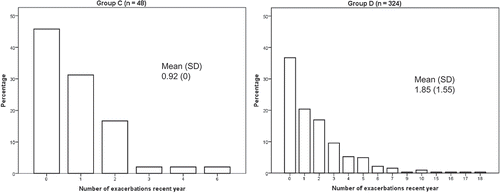
As only patients with COPD stage III–IV were included, 100% of the patients fulfilled the spirometric criterion for a high risk according to the present GOLD classification and were classified as either group C or D. Only 13% had minor symptoms (CAT < 10), and subsequently 87% of the population were classified as group D. In addition, 49% of the patients had frequent exacerbations ().
Figure 1. Reclassification of severity for the patient population according to GOLD 2014 risk evaluation groups A–D
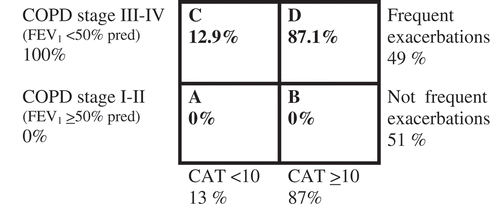
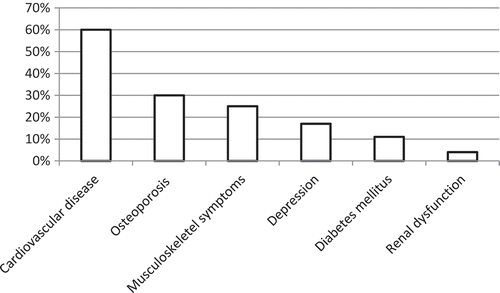
There were no differences in sex, age, BMI, comorbidities or smoking status, although the accumulated exposure to tobacco and exacerbations frequency in the previous year was statistically significantly higher in group D (). In the total population, the prevalence of current smoking was 16%, and the number of comorbid conditions varied from 1 to 4. The most common comorbidity was cardiovascular disease (). The mean exacerbation frequency in the previous year was 1.7 (SD 2.4), but varied from 0 to 18 ().
Table 1. Patient characteristics
Pharmacological treatment and vaccinations
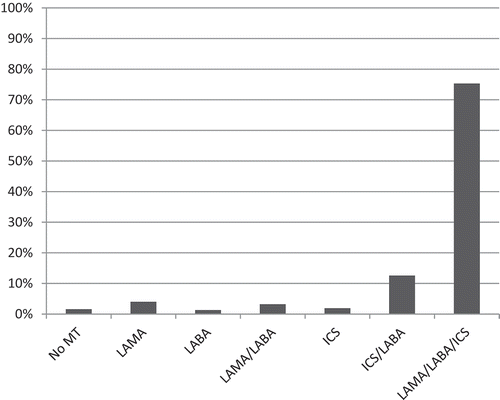
The most common maintenance treatment was triple inhaled therapy with long-acting muscarinic antagonists, long-acting beta-2-agonists and inhaled corticosteroids present in 75.3% of the patients (). Maintenance treatment with only bronchodilators was more common in stage III compared to stage IV, and in group C compared with group D. Maintenance treatment with inhaled corticosteroids with or without bronchodilators was more common in stage IV than in stage III, and in group D than in group C (). Add-on treatment with LTOT was present in 46 patients (12.3%). Roflumilast was prescribed in 27 patients (7.2%) and 30% of these (n = 8) fulfilled the current indications for roflumilast in Sweden (FEV1% pred < 50, frequent exacerbations and chronic bronchitis). As for vaccinations, 75.6% had received influenza vaccination in the previous year and 63% had received pneumococcal vaccination in the previous five years. Influenza and pneumococcal vaccination was more common in group D than C but did not differ between stage III and IV ().
Table 2. Pharmacological interventions by disease severity
Structure and resources in COPD care
According to the structural questionnaires, national guidelines from The National Board of Health and Welfare, the Swedish Medical Products Agency and the Swedish Respiratory Society were most commonly used, in 97% of the respiratory units. Local or regional guidelines were less common (28 and 14%, respectively). The international GOLD document was reported as a base for management in 45% of the units.
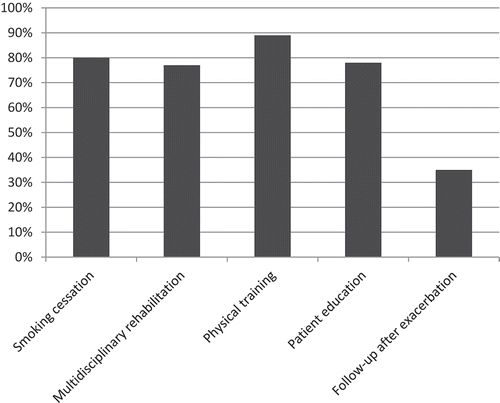
Smoking cessation, multidisciplinary rehabilitation, physical training and patient education were all available at more than 75% of the units. Routine follow-up visits after exacerbations at the respective secondary respiratory units were available in 35% of the participating units (). In multiple linear regression analyses, patients managed at units with access to structured patient education had statistically significant exacerbations in the previous year (). No other significant association between access to resources and patient related outcomes such as exacerbations or health status were found (data not shown).
Table 3. Patient education and exacerbation frequency
Discussion
The first finding of this study is that stage III and IV COPD patients in Swedish secondary care often also suffer from frequent exacerbations and symptoms. By the time of the data collection the Swedish national guidelines of severity classification and treatment recommendations were based only on GOLD 2011 stages I–IV according to lung function measurement, and the present study included patients with stage III and IV. When the patients were reclassified according to GOLD 2014 risk staging, 87% had a severe disease with high risk and symptom burden (group D).
Secondly, most but not all patients had pharmacological treatment with both long-acting muscarinic antagonists, long-acting beta-2-agonists and inhaled corticosteroids, with no difference between group C and D. Treatment with only bronchodilators was more common in group C or lung function stage III, and treatment with inhaled corticosteroids was more common in group D and stage IV. Long-acting muscarinic antagonists [Citation15] as well as inhaled corticosteroids in combination with long-acting beta-2-agonists have a well-documented effect on exacerbations.[Citation16] More recent data have suggested a similar, or even better effect on exacerbations by double bronchodilation as from combined long-acting beta-2-agonists and inhaled corticosteroids.[Citation17] However, we find it reasonable that the small but severely ill group of COPD patients managed in secondary care had optimized treatment with triple inhaled therapy, as this combination has been shown to have an addition effect on exacerbation frequency and symptoms in COPD.[Citation18,Citation19] Roflumilast had a limiting label and subsidization in Sweden at the time of data collection, which may explain its use in only 7% of the patients. Some 22% of the study patients (n = 82) had chronic bronchitis and frequent exacerbations which potentially could have indicated treatment with roflumilast. Among the few patients who were actually prescribed roflumilast, only a third had the documented phenotype and were prescribed roflumilast on an evidence based ground. We believe that the reason for this discrepancy is that physicians not always follow guidelines.
As for pharmacologic prevention with vaccinations, the coverage of influenza vaccination was higher than for pneumococcal vaccination. Influenza vaccination is administrated for free in Sweden, which could explain the difference from pneumococcal vaccination. In our opinion, the difference could also be justified by the much more solid scientific documentation for influenza vaccination than for pneumococcal vaccination in COPD.[Citation20]
The third finding of interest is that access to smoking cessation, multidisciplinary rehabilitation, physical training and education is common, but still not available in all secondary care centers. Access to smoking cessation support, multidisciplinary rehabilitation, physical training and patient education was reported by more than 75% of the units whereas follow-up after exacerbations was only reported by 35% of the units. In comparison, the National COPD Resources and Outcomes Project in UK, including 100 larger sized hospitals, reported access to pulmonary rehabilitation in 89%, education programs in 89%, but full multidisciplinary rehabilitation in only 44%.[Citation21] Physical training was the most common of the investigated resources in Swedish secondary care respiratory units. This is encouraging, as physical training has been shown to improve dyspnea, health related quality of life, physical capacity [Citation22] and, in association to exacerbations, even hospitalization and mortality.[Citation23] Nevertheless, in our opinion physical training should be generally available at secondary care respiratory units for COPD.
Interestingly, access to patient education was statistically significantly associated with a reduced risk for exacerbations. Patient education can be applied in different ways; by lectures for groups of patients or by individual structured discussions.[Citation24,Citation25] One of the major aims with patient education is self-management; disease control through behavior change.[Citation26] We speculate that the association of patient education with lower exacerbation frequency in our study population could be due to achievement of self-management skills. However, development of patient education in a center may also be a general marker of better and more intense management and treatment in all aspects. The finding is consistent with previous studies where a structured education system with the aim of increased self-management was associated with higher HRQL [Citation27] and a reduced risk for hospitalized exacerbations.[Citation28] Exacerbations of COPD are associated with increased decline in lung function and increased mortality, and thus follow-up after exacerbation is also crucial. The fact that a routine with follow-up at respective unit was present in only 35% and that the presence of follow-up was not associated with reduced exacerbation risk, may be related to a well-functioning system with follow-up by asthma/COPD nurses in Swedish primary care. Several Swedish studies have shown that access to or planned follow-up by a specific asthma COPD nurse in primary care is associated with reduced risk for exacerbations.[Citation29–Citation31]
Less than half of the secondary care respiratory units claimed to follow the international GOLD recommendations. In a survey of guideline-based COPD secondary care in Germany, a similar trend with greater adherence to national than international guidelines was observed.[Citation32] We speculate that the preference of national guidelines in our study was due to the fact that during the time of data collection; national treatment recommendations were still based on the previous GOLD 2011 lung function stage I–IV classification, and did not incorporate the new ABCD classification. However, in reality not only lung function but also symptoms and exacerbations were influencing treatment choice.
Strengths and limitations
Strengths of the current study include that it is a multicenter study with patients from almost all respiratory units in Sweden, and that it includes both patient related and structural data. Weaknesses include the fact that treatment information included only pharmacological treatment but not data on whether the individual patients actually received pulmonary rehabilitation or smoking cessation support even if available at the respective units.
Conclusion
We conclude that most GOLD 2011 stage III–IV patients managed at secondary care units for COPD in Sweden have maximized inhaled therapy and high risk disease, even when reclassified to GOLD 2014 A–D groups. Many units have access to smoking cessation, rehabilitation and patient education. Patients managed at units with structured patient education have a lower exacerbation risk.
Acknowledgments
We thank all participating units.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Notes on contributors
Josefin Sundh
Josefin Sundh is a pulmonologist and PhD at the Department of Respiratory Medicine, School of Medical Sciences, Örebro University, Örebro, Sweden. She is a member of the Swedish and European Respiratory Societies. Her research has mainly focused on observational studies in COPD, but also includes studies of asthma, dyspnea and hypoxia.
Christer Janson
Christer Jansson is a pulmonologist and Professor in Respiratory Medicine at the Department of Medical Sciences for Respiratory; Allergy and Sleep Research, Uppsala University, Uppsala, Sweden. He is a member of the Swedish and European Respiratory Societies. His research has focused on asthma and COPD.
Gunnar Johansson
Gunnar Johansson is a general practitioner and Professor Emeritus in General Medicine at the Department of Public Health and Caring Science, Family Medicine and Preventive Medicine, Uppsala University, Uppsala, Sweden. He is a member of The Swedish College of General Practice and the International Primary Care Respiratory Group. His research has focused on clinical and register studies on COPD, asthma and diabetes.
Anders Lindén
Anders Lindén is a pulmonologist, allergologist and Professor in Lung and Airway Research and Head of Unit at the Institute of Environmental Medicine, Karolinska Institutet, Stockholm, Sweden. He is a member of the Swedish, European and American Respiratory Societies, as well as of the European and American Immunological Societies. His research is focused on pulmonary host defence in health and disease, with special attention to the malfunctions of the innate immune response in asthma and COPD.
Claes-Göran Löfdahl
Claes-Göran Löfdahl is a pulmonologist and Professor Emeritus in Respiratory Medicine at the Department of Respiratory Medicine and Allergology, Lund University, Lund, Sweden. He is a member of the Swedish, European and American Respiratory Societies. His research has focused on pharmacology and pathologic mechanisms in COPD and asthma.
Thomas Sandström
Thomas Sandström is a pulmonologist and Professor in Respiratory Medicine at the Department of Public Health and Clinical Medicine, Division of Medicine, Umeå University, Umeå, Sweden. He is a member of the Swedish and European Respiratory Societies. His research has focused on health effects of air pollution, asthma and COPD.
Kjell Larsson
Kjell Larsson is a pulmonologist and Professor in Respiratory Medicine at the Unit for Lung and Airway Research, Institute of Environmental Medicine, Karolinska Institutet, Stockholm, Sweden. He is a member of the Swedish and European Respiratory Societies. His research has focused on clinical, inflammatory and physiologic mechanisms in asthma and COPD and following exposure to organic -dust.
References
- Backman H , Eriksson B , Rönmark E , et al. Decreased prevalence of moderate to severe COPD over 15 years in northern Sweden. Respir Med. 2016;114:1–8. DOI:10.1016/j.rmed.2016.03.013
- Danielsson P , Ólafsdóttir IS , Benediktsdóttir B , et al. The prevalence of chronic obstructive pulmonary disease in Uppsala, Sweden–the Burden of Obstructive Lung Disease (BOLD) study: cross-sectional population-based study. Clin Respir J. 2012;6(2):120–127. DOI:10.1111/j.1752-699X.2011.00257.x
- Torén K , Olin A-C , Lindberg A , et al. Vital capacity and COPD: the Swedish CArdioPulmonary bioImage Study (SCAPIS). Int J Chron Obstruct Pulmon Dis. 2016;11:927–933. DOI:10.2147/COPD.S104644
- Lozano R , Naghavi M , Foreman K , et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095–2128. DOI:10.1016/S0140-6736(12)61728-0
- Sundh J , Janson C , Lisspers K , et al. Clinical COPD Questionnaire score (CCQ) and mortality. Int J Chron Obstruct Pulmon Dis. 2012;7:833–842. DOI:10.2147/COPD.S38119
- Swedish National Board of Health and Welfare . Socialstyrelsens riktlinjer för vård av astma och kroniskt obstruktiv lungsjukdom. Faktadokument och beslutsstöd för prioriteringar. Swedish National Board of Health and Welfare; 2004. Available from http://www.socialstyrelsen.se/english
- Agency SMP . Chronic Obstructive Pulmonary Disease (COPD)- treatment recommendation. 2015 [ updated 2015 Oct 16]. Available from: https://lakemedelsverket.se/upload/halso-och-sjukvard/behandlingsrekommendationer/Kroniskt_obstruktiv_lungsjukdom_KOL_behandlingsrekommendation.pdf
- Global Initiative for Chronic Obstructive Lung Disease . Global strategy for the diagnosis, management, and prevention of Chronic Obstructive Pulmonary Disease. [ updated 2016; cited 2016 Jun 14]. Available from: http://www.goldcopd.com
- Sundh J , Ställberg B , Lisspers K , et al. Co-morbidity, body mass index and quality of life in COPD using the Clinical COPD Questionnaire. COPD. 2011;8(3):173–181. DOI:10.3109/15412555.2011.560130
- Kruis AL , Ställberg B , Jones RCM , . Primary care COPD patients compared with large pharmaceutically-sponsored COPD studies: an UNLOCK validation study. Plos One. 2014;9(3):e90145. DOI:10.1371/journal.pone.0090145
- Sundh J , Johansson G , Larsson K , et al. Comorbidity and health-related quality of life in patients with severe chronic obstructive pulmonary disease attending Swedish secondary care units. Int J Chron Obstruct Pulmon Dis. 2015;10:173–183. DOI:10.2147/COPD.S74645
- Sundh J , Johansson G , Larsson K , et al. The phenotype of concurrent chronic bronchitis and frequent exacerbations in patients with severe COPD attending Swedish secondary care units. Int J Chron Obstruct Pulmon Dis. 2015;10:2327–2334. DOI:10.2147/COPD.S91362
- Wedzicha JA , Seemungal TAR. COPD exacerbations: defining their cause and prevention. Lancet. 2007;370(9589):786–796. DOI:10.1016/S0140-6736(07)61382-8
- Landbo C , Prescott E , Lange P , et al. Prognostic value of nutritional status in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160(6):1856–1861. DOI:10.1164/ajrccm.160.6.9902115
- Tashkin DP , Celli B , Senn S , et al. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359(15):1543–1554. DOI:10.1056/NEJMoa0805800
- Calverley PMA , Anderson JA , Celli B , et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356(8):775–789. DOI:10.1056/NEJMoa063070
- Wedzicha JA , Banerji D , Chapman KR , et al. Indacaterol-glycopyrronium versus salmeterol-fluticasone for COPD. N Engl J Med. 2016;374:2222–2234. DOI:10.1056/NEJMoa1516385
- Welte T , Miravitlles M , Hernandez P , et al. Efficacy and tolerability of budesonide/formoterol added to tiotropium in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009;180(8):741–750. DOI:10.1164/rccm.200904-0492OC
- Lee S-D , Xie C-M , Yunus F , et al. Efficacy and tolerability of budesonide/formoterol added to tiotropium compared with tiotropium alone in patients with severe or very severe COPD: a randomized, multicentre study in East Asia. Respirology. 2016;21(1):119–127. DOI:10.1111/resp.12646
- Schembri S , Morant S , Winter JH , et al. Influenza but not pneumococcal vaccination protects against all-cause mortality in patients with COPD. Thorax. 2009;64(7):567–572. DOI:10.1136/thx.2008.106286
- Stone RA , Harrison BD , Lowe D , et al. Introducing the national COPD resources and outcomes project. BMC Health Serv Res. 2009;9:173. DOI:10.1186/1472-6963-9-173
- McCarthy B, Casey D, Devane D et al. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015 Feb 23;(2):CD003793.
- Puhan MA , Gimeno-Santos E , Scharplatz M , et al. Pulmonary rehabilitation following exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2011 Oct 5;(10):CD005305.
- Stoilkova A , Janssen DJA , Wouters EFM. Educational programmes in COPD management interventions: a systematic review. Respir Med. 2013;107(11):1637–1650. DOI:10.1016/j.rmed.2013.08.006
- Zwerink M , Brusse-Keizer M , van der Valk PD , et al. Self management for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2014 Mar 19;(3):CD002990.
- Bourbeau J , Nault D , Dang-Tan T. Self-management and behaviour modification in COPD. Patient Educ Couns. 2004;52(3):271–277. DOI:10.1016/S0738-3991(03)00102-2
- Koff PB , Jones RH , Cashman JM , et al. Proactive integrated care improves quality of life in patients with COPD. Eur Respir J. 2009;33(5):1031–1038. DOI:10.1183/09031936.00063108
- Benzo R , Vickers K , Novotny PJ , et al. Health Coaching and Chronic Obstructive Pulmonary Disease Rehospitalization. A Randomized Study. Am J Respir Crit Care Med. 2016;194:672–680. DOI:10.1164/rccm.201512-2503OC
- Löfdahl C-G , Tilling B , Ekström T , et al. COPD health care in Sweden - A study in primary and secondary care. Respir Med. 2010;104(3):404–411. DOI:10.1016/j.rmed.2009.10.007
- Sundh J , Österlund Efraimsson E , Janson C , et al. Management of COPD exacerbations in primary care: a clinical cohort study. Prim Care Respir J. 2013;22(4):393–399. DOI:10.4104/pcrj.2013.00087
- Lisspers K , Johansson G , Jansson C , et al. Improvement in COPD management by access to asthma/COPD clinics in primary care: data from the observational PATHOS study. Respir Med. 2014;108(9):1345–1354. DOI:10.1016/j.rmed.2014.06.002
- Glaab T , Vogelmeier C , Hellmann A , et al. Guideline-based survey of outpatient COPD management by pulmonary specialists in Germany. Int J Chron Obstruct Pulmon Dis. 2012;7:101–108. DOI:10.2147/COPD.S27887