ABSTRACT
Background: Omalizumab improves asthma control in patients with uncontrolled severe allergic asthma; however, appropriate patient selection is crucial. Information in this field is sparse.
Objective: We aimed to estimate whether potential omalizumab candidates were appropriately selected according to guidelines, and the clinical effect of omalizumab treatment over time.
Design: We performed a retrospective observational study on adult patients with asthma treated with omalizumab during 2006–2015 at the Department of Respiratory Medicine at Odense University Hospital (OUH), Denmark. Data were obtained from the Electronic Patient Journal of OUH and Odense Pharmaco-Epidemiological Database. Guideline criteria for omalizumab treatment were used to evaluate the appropriateness of omalizumab candidate selection, and the Asthma Control Test (ACT) to assess the clinical effects of omalizumab at weeks 16 and 52 from treatment initiation.
Results: During the observation period, 24 patients received omalizumab, but only 10 patients (42%) fulfilled criteria recommended by international guidelines. The main reasons for not fulfilling the criteria were inadequately reduced lung function, insufficient number of exacerbations, and asthma standard therapy below Global Initiative for Asthma (GINA) step 4–5. Seventeen and 11 patients completed treatment at weeks 16 and 52, with a statistically significant increase in ACT score of 5.1 points [95% confidence interval (CI) 3.1–7.2, p = 0.0001] and 7.7 points (95% CI 4.3–11.1, p = 0.0005), respectively.
Conclusion: Only 42% of the omalizumab-treated patients were appropriately selected according to current guidelines. Still, as omalizumab showed significant improvement in asthma control over time, it is important to keep this drug in mind as an add-on to asthma therapy in well-selected patients.
Introduction
The main goal of asthma management is to achieve and maintain symptom control and normal activity levels, and to minimise the future risk of exacerbations [Citation1,Citation2]. Inadequately controlled asthma affects both patients and society in terms of days lost from work and school, reduced quality of life (QoL), and avoidable healthcare visits and hospitalisations [Citation3]. Since asthma affects around 300 million people worldwide, this is a major public health problem and managing patients with severe asthma, in particular, can be a challenge [Citation4]. Despite treatment with inhaled corticosteroids (ICS) and long-acting β2-agonists (LABA), severe asthma is often uncontrolled and accounts for up to 80% of the total costs of asthma, even though severe asthma represents only 5–10% of the total asthma population [Citation4,Citation5].
Omalizumab is a monoclonal antibody indicated as add-on therapy for patients with severe persistent asthma. Approximately 50% of patients with uncontrolled severe asthma have an immunoglobulin E (IgE)-mediated phenotype [Citation4,Citation6]. IgE plays an important role in allergic asthma and particularly in the acute response to antigens and in the proliferation of airway inflammation [Citation7]. Omalizumab inhibits binding of free IgE to high-affinity receptors on pro-inflammatory cells, which clinically correlates with a reduction in asthma symptoms and exacerbations [Citation8,Citation9].
Clinical trials and observational studies have shown omalizumab to significantly reduce exacerbation rates and frequency of hospitalisations, together with improving both asthma QoL scores and symptom control [Citation4,Citation10–Citation12]. Since uncontrolled severe asthma is a major healthcare problem, it is crucial that potential candidates for omalizumab treatment are selected appropriately, according to current guidelines. Omalizumab is a rather expensive treatment, with a cost per defined daily dose (DDD) of around €50, necessitating good compliance with regular outpatient clinic attendance. Only scant evidence exists on the appropriateness of selection, use, and clinical effectiveness of omalizumab. Hence, the objectives of this study were to assess, first, whether asthma patients treated with omalizumab were selected appropriately according to current guidelines; and secondly, the clinical effect of omalizumab treatment assessed according to changes in asthma symptoms, lung function, asthma control medication, and asthma exacerbations over time.
Methods
Design
We performed a retrospective, observational study in adult patients with severe allergic asthma treated with omalizumab in the Region of Southern Denmark during an observational period from 1 October 2006 to 31 March 2015. Data were analysed using longitudinal and individual repetitive cross-sections according to scheduled follow-ups.
Setting
In the Region of Southern Denmark, examination and treatment of severe asthma, including specialised treatment with omalizumab, are managed at the Department of Respiratory Medicine at Odense University Hospital (OUH). This patient category is primarily assessed in the outpatient clinic, which acts as a secondary and tertiary specialist unit serving a population of nearly one million people aged ≥ 15 years (1 January 2015) [Citation13]. Before receiving omalizumab, all patients go through a preliminary examination about 1 or 2 weeks before the index date, defined as the day of omalizumab initiation, to secure appropriate selection and to determine the correct dose based on weight and IgE level. Omalizumab is indicated only for patients fulfilling the following criteria: age ≥ 6 years; severe persistent asthma; documented positive skin test or in vitro reactivity to a perennial aeroallergens; frequent daytime symptoms or night-time awakenings; asthma exacerbations requiring systemic glucocorticoids despite daily high-dose ICS [or leukotriene receptor antagonist (LTRA)] and LABA; weight of 20–150 kg; total IgE at 30–1500 IU/mL; and for patients aged ≥ 12 years, forced expiratory volume in 1 sec (FEV1) < 80% [Citation1,Citation14–Citation16]. In accordance with the omalizumab European Union (EU) label and guidelines from the Danish Society of Respiratory Medicine, the treatment effect was evaluated after 16 weeks, and if the treatment was continued further evaluation was performed at annual visits [Citation14,Citation16,Citation17].
Data sources
The data for this study were obtained from two clinical databases: the Electronic Patient Journal (EPJ) of OUH and the Odense Pharmaco-Epidemiological Database (OPED).
EPJ
EPJ contains journal data on patients from all admissions and outpatient clinic visits. The data include medication status, pulmonary function tests (PFTs), Asthma Control Test (ACT) score, and blood sample results. Furthermore, EPJ contains information on hospital electronic medical record systems from all regions, providing an overview of a patient’s medical record in relation to a hospital visit.
Since 2006, the Department of Respiratory Medicine at OUH has systematically registered all patients treated with omalizumab, comprising a total cohort of 32 patients.
OPED
Information on reimbursed drug dispensing in the County of Funen has been recorded in the OPED since 1990, and from 1 January 2007 for the entire Region of Southern Denmark (population 1.2 million) [Citation13,Citation18]. Each prescription record includes a person identifier, the date of dispensing, and the brand, active substance, quantity, and form of the drug. The substances and quantities are registered according to the Anatomical Therapeutic Chemical Classification System (ATC) of the World Health Organization and DDD methodology [Citation19]. The indication for treatment and the dosing instruction are not recorded. Drugs not reimbursed and therefore not recorded in the database are over-the-counter drugs and some non-reimbursed prescription drugs, mainly oral contraceptives, hypnotics, sedatives, some antibiotics and intranasal drugs for rhinitis, but also drugs registered only for hospital use, e.g. omalizumab [Citation20].
Study population
Among the cohort of omalizumab-treated asthma patients, only those from whom we received informed written consent were included. All data were anonymised. Owing to treatment evaluation taking place after 16 weeks, only patients fulfilling 16 weeks of treatment were included for objective 2.
For each included patient, EPJ data related to omalizumab treatment (e.g. PFTs, smoking history, ACT score, and IgE levels) were retrieved and indirectly used for manually validating the asthma diagnosis and the appropriateness of omalizumab treatment. The total number of admissions 1 year before omalizumab initiation and documentation of allergy tests were sought and validated through old records, e.g. former admission journals and discharge summaries.
OPED were used to retrieve data on all redeemed individual medication from 1990 to 31 March 2015. Prescriptions redeemed before omalizumab treatment were used to classify comorbidities (comorbidities according to ATC codes, Appendix I). The date of omalizumab initiation was defined as the index date for the individual patient. As a surrogate marker of compliance with asthma controller medication, i.e. ICS (ATC R03BA), LABA (ATC R03AC12 R03AC13), ICS/LABA (ATC R03AK06, R03AK07, R03AK08, R03AK09, R03AK10, and R03AK11), or LTRA (ATC R03DC), we used a definition of having redeemed prescriptions of either ICS and LABA, or LTRA and LABA 6 months before the individual index date.
Outcome variables
Objective 1
The primary outcome was to determine the proportion of appropriate selected patients initiated with omalizumab on the basis of guideline recommendations [Citation16]. We defined severe persistent asthma according to the European Respiratory Society/American Thoracic Society definition of severe asthma, and the criteria regarding frequent daily or nocturnal symptoms were fulfilled when patients had an ACT score below 20 points, equivalent to uncontrolled asthma [Citation5]. The number of prescriptions for systemic glucocorticoids (ATC H02AB) was used as a surrogate marker of asthma exacerbations before and during treatment with omalizumab. Primary compliance to medication (i.e. at least one redeemed prescription for asthma control medication) was measured using the OPED.
Objective 2
Patients completing 16 weeks of omalizumab treatment were included (). The primary outcome was asthma control measured by the use of ACT, a simple quantitative tool for assessing asthma control consisting of a validated five-item questionnaire. Each item is scored on a five-point Likert scale from 1 to 5 (1 = worst; 5 = best), and total scores of 5–19 and 20–25 points reflect uncontrolled and well-controlled asthma, respectively. The minimal clinically important difference (MCID) is 3 ACT points [Citation21]. Asthma control was measured by the ACT at the preliminary examination and at scheduled follow-ups. The latter also applied to secondary outcomes such as changes in PFTs, asthma medication status, and number of exacerbations.
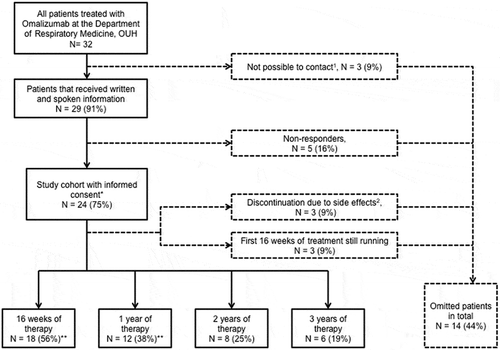
Figure 1. Flowchart for enrolment of patients in the study. The numbers of patients completing 2 and 3 years of therapy were considered too small for analysis. *Patients used for objective 1; **patients used for objective 2. 1Cause of missing contact: death (1), missing contact information (2); 2side effects registered: general physical discomfort (1), arthralgia (1), headache, fatigue and dyspnoea (1)
On the basis of redeemed prescriptions, comorbidities were categorised using the same categorisation as validated by Kuo et al. [Citation22]; however, corticosteroids for systemic use (ATC H02A) were removed from the rheumatic category owing to an essential overlap with treatment of asthma exacerbations.
Data analysis
Using the unique civil registration number (CRN) assigned to every Danish citizen, relevant information was retrieved and linked from the above-mentioned data sources. Outcomes according to the objectives were analysed for each patient according to cross-sectional measurements at the index date, at 16 weeks from treatment initiation, and at 52 weeks of follow-up.
We used a paired t-test to analyse whether the effects on ACT score and PFT measurements were statistically significant. A Q-Q plot for normal distribution was generated before the use of the paired t-test. If a normal distribution was not present, we used the Wilcoxon matched-pairs signed-rank test and Wilcoxon rank-sum test for unmatched data.
Missing values related to objective 1 were registered as ‘criterion not fulfilled’, whereas missing values in relation to objective 2 were treated with pairwise deletion. Statistical analyses were performed using Stata version 13.0 (StataCorp, College Station, TX, USA). A p value < 0.05 was considered statistically significant.
According to Danish law, no ethical approval is needed for register-based studies. The Danish Data Protection Agency (J no. 2008-58-0035) approved the study. All patients gave written informed consent.
Results
In total, 32 patients were treated with omalizumab from 1 October 2006 to 31 March 2015. The flowchart for enrolment is presented in and baseline data are presented in .
Table 1. Baseline characteristics
Objective 1
In total, 24 omalizumab-treated patients (54% women) who had undergone a preliminary examination were included for objective 1 (). Five of these patients (21%) had no redeemed prescriptions for asthma control medication and were therefore categorised as non-compliant with asthma control medication, and in nine patients either data on previous treatment were unavailable (n = 2) or criteria for FEV1, exacerbations, or ACT score were not met. According to guideline recommendations, 10 patients (42%) of the study population fulfilled all criteria for omalizumab treatment. The number of patients fulfilling the criteria remained unchanged when adding primary compliance with control medication (i.e. ICS, ICS/LABA, and LTRA/LABA) [Citation16].
Table 2. Fulfilled omalizumab selection criteria
Objective 2
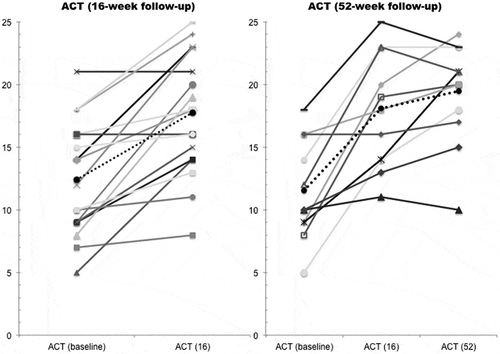
During the observation period, 18 and 12 patients completed 16 and 52 weeks of treatment, respectively. However, one patient was excluded owing to a missing ACT score at the index date. A statistically significant increase in mean ACT score by 5.1 points [95% confidence interval (CI) 3.1–7.2, p = 0.0001] was found when comparing ACT scores from the index date and at 16 weeks’ follow-up, and 11 patients (65%) had a clinically significant increase of ≥ 3 points in total ACT score. For patients completing 52 weeks of treatment, mean ACT scores increased by 7.7 points (95% CI 4.3–11.1, p = 0.0005) and nine (82%) patients had a clinically significant increase in ACT score compared with the index date. illustrates a significant improvement in the proportion of controlled patients, with five (p = 0.03) and seven (p = 0.01) further patients having ACT scores of ≥ 20 after 16 and 52 weeks of omalizumab treatment, respectively.
Figure 2. Changes in Asthma Control Test (ACT) score after 16 and 52 weeks of treatment for 17 and 11 patients, respectively. Each line represents a patient and the dotted line represents the mean ACT score. At the 16 and 52 week follow-up the mean ACT score was 17.5 and 19.3, respectively
Women had a 4.56 point (95% CI 0.84–8.28, p = 0.02) higher increase than men in mean ACT score from the index date to the 16 week follow-up, and the same tendency was present after 52 weeks, with a 6.39 point mean ACT score increase (p > 0.05) for women. The ACT mean scores increased in the two follow-ups in both genders, but only women had a statistically significant increase.
The mean FEV1% predicted for patients completing 52 weeks of omalizumab treatment showed a slight change from the index date to 16 and 52 weeks of follow-up, corresponding to 11 percentage points (95% CI 1–20, p = 0.03) and 10 percentage points (95% CI 0–21, p = 0.05), respectively. The matching changes in mean FEV1 were 0.31 L and 0.29 L, respectively. The remaining PFT measurements showed no significant changes, and are illustrated in together with mean biannual exacerbation rates for the patients completing 52 weeks of treatment.
Table 3. Development in mean pulmonary function test (PFT) measurements and mean exacerbation rates for patients completing 52 weeks of treatment
The mean number of exacerbations decreased throughout the 52 weeks. However, one patient was responsible for the majority of the total number of exacerbations, corresponding to seven out of 11 (64%) and four out of seven (57%) exacerbations, respectively, after 16 and 52 weeks of omalizumab treatment. Furthermore, we found that the frequency of annual asthma exacerbations reduced by 28% or an average of 0.64 (p > 0.05) exacerbations compared to the year before the index date (data not shown).
No significant difference was found in asthma control between appropriately selected versus non-appropriately selected patients when stratifying for variables such as correct omalizumab dose, obesity [body mass index (BMI) > 30 kg/m2], smoking status, and compliance with asthma control medication.
Discussion
The main finding of this study was that only 42% of the patients with severe asthma treated with omalizumab had been appropriately selected according to current guidelines [Citation1,Citation14–Citation16]. Despite this, omalizumab had a significant effect on asthma control, with an increase in mean ACT score of 5.1 and 7.7 at 16 and 52 weeks’ follow-up, respectively, and with the highest mean ACT scores in women and no significant increase in men. Although not statistically significant, a trend towards a reduction in numbers of exacerbations was observed after 52 weeks of omalizumab treatment compared to before initiation of omalizumab treatment. In addition, obesity seemed to be a general trait among all omalizumab-treated patients.
According to objective 1, previous studies have not paid much attention to the appropriateness of selecting patients for omalizumab treatment in clinical settings, which is why there is no direct frame of reference. Changes in the selection criteria during the observation period could, however, have had a negative influence on the overall proportion of well-selected patients, as the criteria used in this study are based on current guideline recommendations [Citation1,Citation14–Citation16]. Thus, comparing the number of treated patients and the possible number of patients eligible for omalizumab treatment seems somewhat contradictory. The current selection seems inadequate owing to a low number of omalizumab-treated patients fulfilling the selection criteria, and more patients are expected to be treated with omalizumab according to asthma prevalence. In Denmark, the prevalence of asthma in adults is around 7%, of whom 8% can be classified as having severe asthma [Citation23,Citation24]. If at least 50% have allergic asthma, 2,800 adults are likely to have severe allergic asthma in the Region of Southern Denmark. This number may be a rough estimate, but the number of treated patients in our study and the expected number of patients treated with omalizumab differ. This difference is probably due to multiple factors, including attitudes towards treatment, symptom neglect among patients, socio-economic factors such as disposable income, the treating physician’s knowledge of severe asthma and available insight into the guidelines, deselection of treatment, and bottlenecks in referral to tertiary asthma centres [Citation25,Citation26]. A major study by Buhl et al. supports this explanation [Citation27].
The results of objective 2 are consistent with findings from previous studies documenting that treatment with omalizumab in patients with severe persistent asthma increases asthma control and reduces exacerbation rates [Citation4]. A large, international, observational study of omalizumab use in 943 patients found similar results according to ACT score, with an increase of 6.1 on average after 12 months of treatment, and mean ACT scores at the index date and after 12 months of 13.0 and 19.1, respectively [Citation12]. However, this study did not aim to assess the appropriateness of selecting candidates for omalizumab. Since the MCID is 3, the increase in ACT score indicates that omalizumab improved asthma control to a level of clinical significance. Because of the small study population, we had very few patients with admissions due to asthma, so it was not possible to perform any significant analysis on the effectiveness of omalizumab in reducing hospitalisations due to asthma.
Women had better asthma control than men at both 16 and 52 weeks. The association between gender and asthma control may be confounded by the higher BMI among women (BMI: women 33 kg/m2 and men 28 kg/m2; data not shown), as obesity is a known predictor of poor asthma control [2014,Citation29]. However, female gender was shown to be associated with worse asthma control in a Spanish cross-sectional study [Citation29].
It is well documented that obesity and uncontrolled asthma are positively associated [Citation30,Citation31]. We found a high mean BMI of nearly 31 kg/m2 in our study population, corresponding to obesity, and although our data cannot be used to explore for causality, this could be a potential explanation for a requirement of omalizumab as add-on therapy to achieve asthma control among these patients. However, evidence to support the benefit of omalizumab on asthma control in obese patients is hard to come by, as most studies investigating the effectiveness of omalizumab have only registered weight, not BMI, making consistent comparisons difficult [Citation12,Citation32,Citation33].
Strengths and limitations
The main strength of the study was the inclusion of all omalizumab-treated patients from a tertiary referral asthma centre covering one million inhabitants aged ≥ 15 years, and not least the manual validation of all data from EPJ, including the asthma diagnosis, sensitisation to perennial aeroallergens, omalizumab treatment, IgE level, BMI, and smoking status. Another strength was the use of electronic pharmacy records, with a high level of completeness, to account for patients’ medication use [Citation34]. As prescription data from OPED were extensive and records completely covered all dispensed drugs, we avoided the problem of recall bias compared with self-reporting, where patients with a chronic disease tend to overestimate their level of compliance [Citation35]. Furthermore, assessing prescription records retrospectively added an additional advantage to our study, since it removed the Hawthorne effect, often seen when patients under observation improve in their adherence to medication. Nonetheless, prescription data account only for primary and not for secondary compliance with medication (i.e. the patient actually takes the medicine) [Citation20]. In this way, valid information on real drug use would have been preferable, e.g. obtained by directly observed therapy or measurement of concentrations of a drug or its metabolite in the blood or urine. However, such approaches have major disadvantages in that they can be rather difficult to administer, expensive, and invasive for the patient.
We may have underestimated the proportion of appropriately selected patients, since missing values in objective 1 were treated as though the criterion was not fulfilled. This leaves a risk of differential misclassification according to OPED data and ACT score. There is also a risk of underestimating exacerbation rates, as patients may have redeemed a large quantity or already have a supply of systemic glucocorticoids at home and could thereby be self-administering when symptoms deteriorate. To obtain a more accurate estimate of exacerbation rates and compliance with asthma control medication, calculation of DDDs could have been an option. The chosen criteria in this retrospective study were based on existing guideline criteria from the European Medicines Agency (EMA), the UK National Institute for Health and Care Excellence (NICE), and the Danish Society of Respiratory Medicine, with only two minor differences. First, in the mentioned guidelines an ACT score < 20 was not an inclusion criterion for omalizumab treatment [Citation14–Citation16]. In our cohort, this criterion excluded four patients (20 patients included versus 24 with informed consent), and if this criterion was omitted three out of the four excluded patients would have fulfilled all the other inclusion criteria, leaving the total number of patients fulfilling all criteria at 13 (54.1%). The second difference was the exacerbation rate. This study included patients with one or more annual exacerbations, whereas NICE and EMA refer to ‘multiple exacerbations’, meaning more than one [Citation14,Citation15]. If the ‘multiple exacerbation’ criterion was used in our cohort and defined as more than two annual exacerbations, this would exclude five of the 19 patients registered with exacerbations, leaving the total number of patients fulfilling all criteria at five (20.8%). Therefore, the applied and potentially restrictive ACT criterion in our cohort was very likely to have been be counterbalanced by the applied exacerbation criterion. Hence, we do not think that patients were excluded from omalizumab treatment differently from other asthma centres using the above-mentioned guideline criteria, and as such we find that the selected patients reliably represent omalizumab candidates outside our region and country.
Another limitation of our study was the lack of power owing to the small study population. However, since we included all patients treated with omalizumab in the Region of Southern Denmark, the only option to enhance power would be to conduct a multicentre study. We had an acceptable inclusion response rate of 75%, but it still entails a risk of selection bias. As the study was retrospective and did not require intervention, responders and non-responders to omalizumab treatment should have had equal incentive to provide informed consent. However, responders might have been more enthusiastic about participating (responders n = 24 versus non-responders n = 5) (), which could have caused a risk of healthy volunteer bias, a subtype of selection bias.
Conclusion
This study found that only 42% of the patients with severe asthma treated with omalizumab were appropriately selected according to current guidelines. Yet, omalizumab is an important add-on treatment option for well-selected asthma patients, as demonstrated by a significant improvement in asthma control. Future studies should focus on how to improve the selection of patients for omalizumab treatment and encourage identification of specific determinants of patients who are likely to benefit from the treatment, rather than the use of predetermined baseline characteristics.
Author contributions
LN validated the data, performed the analysis, interpreted the data, and wrote the first draft of the article. JRD designed the study and wrote the protocol draft, helped with interpretation of data, and made critical revisions to the manuscript for important intellectual content. DPH helped with data analysis, and made critical revisions to the manuscript for important intellectual content. HM provided input to the article, helped with interpretation of data, and made critical revisions to the manuscript for important intellectual content. All authors had full access to all of the data (including statistical reports and tables) in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis. All authors have approved the final version. JRD is the guarantor.
Acknowledgements
We wish to thank Professor MD DMSci Jesper Hallas and MD Morten Rix Hansen from Clinical Pharmacology, Institute of Public Health, Faculty of Health Sciences, University of Southern Denmark for access to data from OPED. Furthermore, we thank the nurses at the asthma unit at the Department of Respiratory Medicine, Odense University Hospital, for their kind help in obtaining clinical data on omalizumab-treated patients.
Disclosure statement
LN and HM declare no competing interests. DPH reports personal fees from AbbVie, outside the submitted work. JRD has received fees from Boehringer Ingelheim, Novartis, TEVA, AstraZeneca, and Norpharma, outside the submitted work.
Additional information
Funding
Notes on contributors
Leo Nygaard
Leo Nygaard , MD, completed his medical degree from the University of Southern Denmark in 2016. Leo has been working as locum resident at the Department of Respiratory Medicine, OUH, and is currently well under way with his internship.
Daniel Pilsgaard Henriksen
Daniel Pilsgaard Henriksen, MD, PhD, completed his medical degree from the University of Southern Denmark in 2009, and his PhD in sepsis epidemiology from the department of Clinical Research, University of Southern Denmark in 2014. Daniel is currently a specialist registrar in clinical pharmacology, working at the Department of Geriatric Medicine, Odense University Hospital. Daniel is a board member of the Danish Society for Pharmacoepidemiology, works part-time as a clinical assessor at the Danish Medicines Agency. Daniel is employed as an external associate professor at the Department of Clinical Medicine, University of Southern Denmark where he teaches clinical pharmacology.
Hanne Madsen
Hanne Madsen, MD, PhD, completed her medical degree from the University of Southern Denmark in 1993 and has a PhD within clinical pharmacology. Hanne is medical specialist in clinical pharmacology and in internal and respiratory medicine, working as head of department at the Department of Respiratory Medicine, Odense University Hospital, Denmark since 2014. Her main research focus is asthma.
Jesper Rømhild Davidsen
Jesper Rømhild Davidsen, MD, PhD, completed his medical degree from the University of Southern Denmark in 2002, and his PhD in asthma pharmacoepidemiology from the Research Unit of General Practice, University of Southern Denmark in 2011. Jesper is a consultant in internal and respiratory medicine at the South Danish Center for Interstitial Lung Diseases at Department of Respiratory Medicine, Odense University Hospital, Denmark. Jesper is Research Associate Professor at Institute of Clinical Research, University of Southern Denmark, Denmark. Jesper's main research area is interstitial lung diseases, lung transplantation, and lung ultrasound.
References
- Global Initiative for Asthma (GINA) . Global strategy for asthma management and prevention 2016. [ updated 2016]. Available from: http://www.ginasthma.org
- Reddel HK , Bateman ED , Becker A , et al. A summary of the new GINA strategy: a roadmap to asthma control. Eur Respir J. 2015;46(3):1–9.
- Norman G , Faria R , Paton F , et al. Omalizumab for the treatment of severe persistent allergic asthma: a systematic review and economic evaluation. Health Technol Assess. 2013;17(52):1–342.
- Normansell R , Walker S , Milan SJ , et al. Omalizumab for asthma in adults and children. Cochrane Database Syst Rev. 2014;1:Cd003559.
- Chung KF , Wenzel SE , Brozek JL , et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43(2):343–373.
- Fanta CH. Asthma. N Engl J Med. 2009;360(10):1002–1014.
- Chung KF. Targeting the interleukin pathway in the treatment of asthma. Lancet. 2015;386(9998):1086–1096.
- Kuhl K , Hanania NA. Targeting IgE in asthma. Curr Opin Pulm Med. 2012;18(1):1–5.
- Holgate S , Smith N , Massanari M , et al. Effects of omalizumab on markers of inflammation in patients with allergic asthma. Allergy. 2009;64(12):1728–1736.
- Humbert M , Beasley R , Ayres J , et al. Benefits of omalizumab as add-on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): INNOVATE. Allergy. 2005;60(3):309–316.
- Hanania NA , Alpan O , Hamilos DL , et al. Omalizumab in severe allergic asthma inadequately controlled with standard therapy a randomized trial. Ann Intern Med. 2011;154(9):573–582.
- Braunstahl GJ , Chen CW , Maykut R , et al. The eXpeRience registry: the ‘real-world’ effectiveness of omalizumab in allergic asthma. Respir Med. 2013;107(8):1141–1151.
- Statistics Denmark . [ cited 2015 Apr 30]. Available from: http://www.dst.dk.proxy1-bib.sdu.dk:2048/da/
- European Medicines Agency . [ cited 2015 May 20]. Available from: http://www.ema.europa.eu/ema/
- National Institute for Health and Clinical Excellence (NICE) . Technology appraisal guidance 278: omalizumab for treating severe persistent allergic asthma (review of technology appraisal guidance 133 and 201). 2013. Available from: https://www.nice.org.uk/guidance/ta278
- Danish Society of Respiratory Medicine . [ cited 2015 May 14]. Available from: http://www.lungemedicin.dk
- Bousquet J , Siergiejko Z , Swiebocka E , et al. Persistency of response to omalizumab therapy in severe allergic (IgE-mediated) asthma. Allergy. 2011;66(5):671–678.
- Davidsen JR , Hallas J , Sondergaard J , et al. Association between prescribing patterns of anti-asthmatic drugs and clinically uncontrolled asthma: a cross-sectional study. Pulm Pharmacol Ther. 2011;24(6):647–653.
- WHO Collaborating Centre for Drug Statistics Methodology . Guidelines for ATC classification and DDD assignment 2015. Oslo; 2014.
- Gaist D , Sorensen HT , Hallas J . The Danish prescription registries. Dan Med Bull. 1997;44(4):445–448.
- Schatz M , Kosinski M , Yarlas AS , et al. The minimally important difference of the asthma control test. J Allergy Clin Immunol. 2009;124(4):719–723e1.
- Kuo RN , Dong YH , Liu JP , et al. Predicting healthcare utilization using a pharmacy-based metric with the WHO’s anatomic therapeutic chemical algorithm. Med Care. 2011;49(11):1031–1039.
- Skadhauge LR , Baelum J , Siersted HC , et al. [The occurrence of asthma among young adults. A population-based study in five west Danish counties]. Ugeskr Laeger. 2005;167(6):648–651.
- von Bulow A , Kriegbaum M , Backer V , et al. The prevalence of severe asthma and low asthma control among Danish adults. J Allergy Clin Immunol Pract. 2014;2(6):759–767.
- Apter AJ , Boston RC , George M , et al. Modifiable barriers to adherence to inhaled steroids among adults with asthma: it’s not just black and white. J Allergy Clin Immunol. 2003;111(6):1219–1226.
- Davidsen JR , Sondergaard J , Hallas J , et al. Impact of socioeconomic status on the use of inhaled corticosteroids in young adult asthmatics. Respir Med. 2011;105(5):683–690.
- Buhl R , Marco AG , Cohen D , et al. Eligibility for treatment with omalizumab in Italy and Germany. Respir Med. 2014;108(1):50–56.
- Sivapalan P , Lange P , Ulrik CS . [Obese asthma patients have poorer asthma control.]. Ugeskr Laeger. 2015;177(24):1146–1150.
- Hermosa JL , Sanchez CB , Rubio MC , et al. Factors associated with the control of severe asthma. J Asthma. 2010;47(2):124–130.
- Lavoie KL , Bacon SL , Labrecque M , et al. Higher BMI is associated with worse asthma control and quality of life but not asthma severity. Respir Med. 2006;100(4):648–657.
- Boulet LP , Franssen E . Influence of obesity on response to fluticasone with or without salmeterol in moderate asthma. Respir Med. 2007;101(11):2240–2247.
- Brusselle G , Michils A , Louis R , et al. “Real-life” effectiveness of omalizumab in patients with severe persistent allergic asthma: the PERSIST study. Respir Med. 2009;103(11):1633–1642.
- Schumann C , Kropf C , Wibmer T , et al. Omalizumab in patients with severe asthma: the XCLUSIVE study. Clin Respir J. 2012;6(4):215–227.
- Sorensen HT , Steffensen FH , Ejlersen E , et al. Research in the Danish health service system: completeness and validity of prescription data, illustrated by analysis of utilization of oral anticoagulants. Int J Risk Saf Med. 1995;7(1):33–41.
- Osterberg L , Blaschke T . Adherence to medication. N Engl J Med. 2005;353(5):487–497.
