ABSTRACT
Context: Patients with chronic obstructive pulmonary disease (COPD) have a high symptom burden and reduced quality of life. There is an increasing attention on palliation for patients with COPD. Recognition of symptoms is a prerequisite for palliation.
Objectives: We aim to investigate the extent to which symptoms in patients with COPD are recognized in the documentation of the health professionals, indicated in ‘Doctors Symptom Recognition Rate’ (DSR), ‘Nurses Symptom Recognition Rate’ (NSR) or ‘Doctors and/or Nurses Symptom Recognition rates ’(DNSR) as a team, respectively.
Methods: Patients with COPD (n = 40) admitted in two respiratory units, responded within 48 h on two symptom-screening-tools that access quality of life; COPD assessment test (CAT) used for the treatment of COPD and EORTC-QLQ-C15-PAL used for palliation in patients with cancer. Patient-described symptomatology was compared to the symptoms as recognized in the documentation of doctors and/or nurses.
Results: There was a significant discrepancy between the symptomatology indicated by patients with COPD on CAT and EORTC-QLQ-C15-PAL, and the degree by which it was recognized in the medical records indicated in DSR or NSR. In 30 out of 44 items DSR or NSR were < 70%. There was a significant difference between DNSR versus DSR or NSR, respectively, in 19 out of 22 items.
Conclusion: A team-based symptom recognition DNSR is superior when compared to DSR or NSR.
Team-based systematic screening is suggested as a pathway to increase symptom recognition in patients with COPD. Increased rates of symptom recognition may improve symptom alleviation and thus palliation.
Introduction
Chronic obstructive pulmonary disease (COPD) is highly prevalent and is worldwide associated with high morbidity and mortality [Citation1–Citation4]. A global estimate from 2016 indicates that about 65 million people live with COPD an amount that is expected to increase as the population ages [Citation5,Citation6]. More than 3 million people died of COPD in 2015 which amounts to approximately 5% of global deaths that year [Citation5]. Currently COPD is the fourth leading cause of death [Citation7] and the World Health Organization (WHO) projects it to be the third leading source of mortality by 2020 [Citation5].
Patients with COPD often experience long disease trajectories characterized by a substantial burden of symptoms, impaired functional status and thereby reduced quality of life [Citation8]. The course of disease is unpredictable due to exacerbations, a decline in respiratory condition in addition to normal day-to-day changes, that are associated with an increased risk of dying [Citation5,Citation6,Citation9–Citation13]. Literature indicates that patients with COPD lives with a symptom burden comparable to patients with lung cancer in the advanced stage of the disease [Citation14,Citation15].
Palliative care aims to alleviate symptoms and thus increase quality of life for patients and their families [Citation6,Citation16]. Patients with advanced COPD benefit equally from palliative care compared to patients with malignant diseases [Citation17]. Despite of this, palliative care in a specialized unit is offered to patients with COPD to a lesser extend than to patients with a malignant disease [Citation15,Citation18]. Few existing studies evaluate the effect of palliative care outside a specialized unit directed at patients with COPD [Citation12,Citation15,Citation19–Citation22].
Currently, there is a growing attention upon palliative care to patients with life-threatening non-malignant diseases as COPD. Palliation has historically been linked to the symptomatology experienced by patients with cancer. It is presumed within oncology that doctors’ and nurses’ symptom recognition is a fundament for the palliative care offered, based on the assumption that if a symptom is not recognized and documented by health professionals, it is unlikely that it will be treated. Studies conducted within a population of patients with cancer shows a discrepancy between symptoms reported by patients and the extent to which they are acknowledged by health professionals [Citation23,Citation24]. This discrepancy reduces chances of symptom alleviation and subsequent increased quality of life [Citation23–Citation25]. Systematic screening for symptoms has been suggested as a way to ensure palliative care in specialized units [Citation23–Citation26,Citation27].
The first step towards ensuring palliative care to patients with life-threatening non-malignant disease, must be to make sure that their symptoms are recognized and documented by health professionals in the medical records.
To the best of our knowledge a validated questionnaire or screening-tool reflecting the palliative symptoms of patients with COPD does not exist, nor do studies elucidating if symptoms are documented.
The aim of this study was to investigate the degree to which symptoms described by admitted patients with COPD through Patient Reported Outcome (PRO) were recognized and documented by health professionals.
Since palliative care aims to alleviating symptoms, the attention of this study was put on symptoms with high intensity and thus a potential for alleviation.
In line with previous studies we wish to estimate ‘Doctor’s Symptom Recognition percentage’ (DSR) [Citation24] and ‘Nurse’s Symptom Recognition percentage’ (NSR) [Citation23], i.e., – the extend to which symptoms with high intensity experienced by admitted patients with COPD and described by either COPD assessment test (CAT) [Citation28] or European Organisation for Research and Treatment for Cancer’s questionnaire reflecting palliative symptoms (EORTC-QLQ-C15-PAL) [Citation29] are recognized by health professionals.
Methods
A prospective survey within a cross-sectional setting was conducted obtaining data from patients admitted with COPD in respiratory units in two different hospitals located in the capital region of Denmark. According to The Ethics Committee (www.nvk.dk) in Denmark, the Biomedical Research Ethics Committee System Act does not apply to survey and qualitative studies in general nor the present study. Participants are referred to by pseudonyms and written informed consents were obtained from all patients before participation.
Patient selection
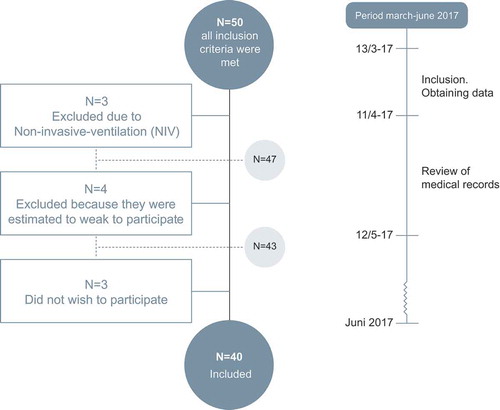
Patients were included consecutively through cluster-sampling from 13 March 2017 to 11 April 2017 from two respiratory units located in different parts of Copenhagen. Inclusion every second day from each hospital if patients had been admitted less than 48 h until n = 40.
Eligible criteria of inclusion were:
-Diagnosis of COPD according to Global initiative for chronic Obstructive Lung Disease (GOLD)[Citation6] criteria, age ≥ 18 years, Danish speaking and able to give an informed consent according to Danish law.
Exclusion criteria were:
-If interviewer assessed patients to weak to participate in interview, had cognitive deficits and/or a cancer diagnosis.
In total 40 patients were included, n = 4 were estimated to weak to participate, n = 3 were treated with acute non-invasive-ventilation increasing the risk of cognitive deficits and n = 3 declined participation ().
Measures
Demographic and disease-related data were obtained from medical records. Patients reported symptoms by CAT [Citation28] and EORTC-QLQ-C15-PAL [Citation29]. Patient reported outcomes (PRO-data) were all collected within first 48 h of admission.
COPD assessment test (CAT)
CAT [Citation28] was designed to reflect the impact of COPD on patients’ health status [Citation30]. CAT was developed based on a population containing all degrees of COPD (n = 1503) [Citation30]. Through item response theory (IRT) eight items were identified each covering a symptom. Intensity of symptoms are measured on a rating-scale ranging from 0 to 5 [Citation31] giving CAT a max score of 40. A change of two units is accepted as minimum clinically significant change [Citation32].
Scorings-algorithms are developed transforming raw-data from an expression of symptom intensity into effects on patients health [Citation33].
According to GOLD classification a CAT-score> 10 units is interpreted as symptomatic COPD [Citation6]. CAT-scores are assessed when patients are categorized based on symptoms and exacerbations in GOLD classification (A-D).
EORTC-QLQ-C15-PAL
EORTC-QLQ-C30 [Citation34] resamples HRQoL in patients with advanced, incurable and symptomatic cancer with a median life expectancy of a few months [Citation35]. EORTC-QLQ-C30 is extensively validated for patients with cancer [Citation36–Citation38] and the use of it is supported by standardized procedures for scores [Citation39] and evidence for interpretation of scores [Citation39,Citation40]. EORTC-QLQ-C15-PAL[Citation29] has become a shortened version of EORTC-QLQ-C30 through IRT preserving item-properties and procedures for scores [Citation35,Citation41–Citation43].
EORTC-QLQ-C15-PAL consists of 15 items of which 14 items are measured on a rating-scale ranging from 1 to 4 equivalent to: 1 = ‘not a problem’, 2 = ‘ a little’, 3 = ‘quite a bit’ and 4 = ‘very much’. Item 15 is rating quality of life on a scale ranging from 1 to 7, 7 being highest quality of life. Functional scales are represented by item 1–3, symptom scales by item 4–14 while quality of life is represented by item 15. Raw-data rated from 1 to 4 can be transformed into a Likert-scale ranging from 0 to 100 ()
Obtaining pro-data
With an awareness that symptomatology changes according to patients being in a stable phase at home or in a acute phase during the first days of hospitalization, all patients were instructed to answer CAT and EORTC-QLQ-C15-PAL according to current symptoms and symptoms within the last seven days.
To minimize missing data and to increase data completeness all PRO-data was obtained through interviews and administrated by a health professional. To ensure consistency all interviews were conducted by first author.
Extracting data from medical records
To identify if symptoms reported by patients were recognized by health professionals we constructed a checklist containing 23 items based on the two questionnaires: 8 scales from CAT and 15 from EORTC-QLQ-C15-PAL.
Symptom recognition of health professionals was registered for doctors and nurses, respectively, and was reviewed qualitatively. Braden-score and ‘functionality and activity’- screenings were both a part of daily documentation for the nurses in both units and were obtained as recognition of functionality scales item 1–3 on EORTC-QLQ-C15-PAL and item 5 ‘activities home’ on CAT.
To optimize objectivity and minimize bias review of documentation was blinded to PRO-data. If a symptom was recognized, date and citation were noted on the checklist to secure validity through transparency.
Data validation
To validate the data an independent reviewer conducted same procedure, as described above, in a random sample of n = 14(35%) of the 40 included medical records and 87.4% of consensus was found.
A rate of 87.4% of consensus in data registration is accepted as high validity of data.
Statistics
Categorization
DSR and NSR will be calculated as the percentage of symptoms described by patients with intensities above the cut-off recognized by health professionals in the medical records.
As an alternative to single recognition by DSR or NSR is a team-based recognition defined as ‘Doctors and/or Nurses Symptom Recognition rates’ (DNSR).
A recognition rate > 70% is accepted as a threshold providing a minimum basis for symptom alleviation in relation to palliative care. For this purpose it is necessary to define which responses describe a symptom with potential for alleviation, i.e., a cut-off-value for symptoms according to CAT and EORTC-QLQ-C15-PAL, respectively
Cut-off-values for symptoms rated on CAT
A score of two units in CAT as a cut-off-value for a symptom corresponds with the minimal clinical difference of two units described by jones et al [Citation31]. Subsequently, this study accepts a score of two units in CAT as a symptom and score above two units as high intensity symptoms.
Cut-off-values for symptoms rated on EORTC-QLQ-C15-PAL
In alliance with previous studies [Citation23,Citation24], this study also accepts a symptom intensity of 2 in EORTC-QLQ-C15-PAL as a threshold and proclaims it our cut-off-value. All intensities> 2 will be regarded as high intensity.
Equivalence and correspondence between CAT and EORTC-QLQ-C15-PAL
Previous studies have converted CAT and EORTC-QLQ-C30 into scales ranging from 0 to 100 [Citation23,Citation24,Citation32].
If we divide 100 with 6 categories on CAT’s Likert-scale (0, 1, 2, 3, 4 and 5), then each category equals to 16.67%. If we accept a cut-off-value of 2 on the Likert-scale then it corresponds to 3 units on the Likert-scale (0, 1, 2) meaning; 3 × 16.67% = 50% .
If we convert the Likert-scales of EORTC-QLQ-C15-PAL into 0–100% and divide it by the four categories (1, 2, 3 and 4), we find that each category equals to 25%. If we accept a cut-off-value of 2 on the Likert-scale then it corresponds to 2 × 25% = 50%.
On this basis we argue that a symptom intensity of 2 on CAT is equivalent to and corresponds with a symptom intensity of 2 on EORTC-QLQ-C15-PAL. From this point and onwards interest will be on data describing symptoms with intensities above 2 i.e., (3, 4 and5) and thus potential for alleviation and hereby palliative care.
Patient’s scores on CAT and EORTC-QLQ-C15-PAL were cross-tabulated against the dichotomous outcome of whether or not a symptom was recognized in the medical records of the same patient by doctors or nurses, respectively. Chi-square-test calculated p-values to describe if symptom recognition was significantly different doctors versus nurses, and to describe if a team-based recognition was significantly different to doctors’ or nurses’ recognition, respectively. p < 0.05 I accepted as significant.
Global QOL on EORTC-QLQ-C15-PAL was not interpreted as a symptom and was excluded from cross-tabulation. Global QOL is shown as a mean with a range (min;max) and SD.
Results
The demographic data of the population are shown in .
Table 1. Demographic data and FEV 1, FVC and FEV1/FVC.
All included patients n = 40 answered all items on both questionnaires.
n = 40 Patients described Global QOL on a scale with a range from 1 to 7 giving a mean = 3, 13(1;7) and SD = 1,522.
In 1 out of 40 reviews, QOL was recognized by doctors and nurses in the medical records corresponding to DSR 2.5% and NSR 2.5%.
Symptoms with high intensity described by patients admitted with COPD.
shows the number of patients who experienced high symptom intensity corresponding to a raw-score> 2.
Table 2. Number of patients who experienced high symptom intensity corresponding to a raw-score> 2.
Table 3. Doctors’ symptom recognition (DSR) and nurses’ symptom recognition (NSR) shown as %.
Table 4. Recognition rates of symptoms with high intensity documented in the medical records.
CAT-score’s converted to percentage’s (Np> 2 CAT/N) × 100 shows: ‘Cough’ 65%, ‘Phlegm’ 55%, ‘Chest feels tight’ 25%, ‘Breathless’ 90%, ‘Activities home’ 82.5%, ‘Confident’ 30%, ‘Sleep’ 47.5% and ‘Energy’ 90%.
EORTC-QLQ-C15-PAL-scores converted to percentages (Np> 2 EORTC-QLQ-C15-PAL/N)x 100 shows: ‘Short walk outside’ 67%, ‘bed-chair’ 60%, ‘Help-activities of daily living’ 17%, ‘short of breath’ 75%, ‘Pain’ 27%, ‘Trouble sleeping’ 42.5%, ‘Felt weak’ 85%, ‘Appetite’ 47.5%, ‘Nauseated’ 12.5%, ‘Constipated’ 17.5%, ‘Tired’ 82.5%, ‘Pain daily activities’ 17.5%, ‘Tense’ 42.5% and ‘Depressed’ 17.5%.
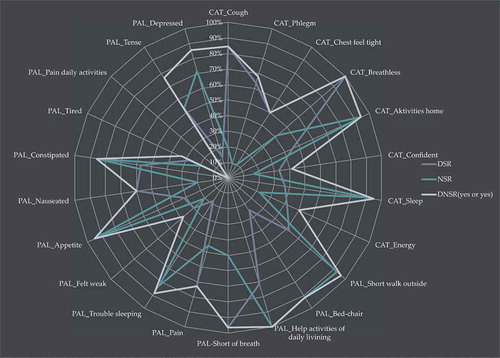
Analysis reflected in shows that 14 out of 44 items reflecting symptoms with high intensity potential for alleviation were recognized in the medical records by rates> 70%: DSR PAL ‘help activities of daily living’ 71%, DSR PAL ‘Pain’ 73%, DSR PAL ‘Short of breath’ 97%, DSR CAT ‘Cough’ 85%, DSR CAT ‘Breathless’ 100%, NSR PAL ‘Trouble sleeping’82%, NSR PAL ‘Constipated’ 86%, NSR CAT ‘Sleep’ 89%, NSR PAL ‘Depressed’ 71%, NSR PAL ‘Short walk outside’ 93%, NSR PAL ‘Bed/chair’ 92%, NSR PAL ‘help activities of daily living’ 100%, NSR PAL ‘Appetite’ 95% and NSR CAT ‘ Activities at home’ 94%.
Main symptoms of patients admitted with COPD were dyspnoea, exemplified by CAT ”breathless” 90% and PAL ‘short of breath’ 75%.
Symptoms of high intensity, with a prevalence> 70% described by admitted patients with COPD apart from their main symptom dyspnoea were: CAT ‘Activities at home’ 82%, CAT ‘energy’ 90%, PAL ‘Felt weak’ 85% and PAL ‘Tiered’ 82.5%. Just 1 of these symptoms was recognized with a rate> 70% (NSR CAT ‘Activities home’ 94%) the rest were recognized by rates ranging between 18% and 45% ().
Table 4 shows that in 9 out of 22 items, DSR were significantly lower (p < 0.05) when compared to DNSR (yes or yes). In 11 out of 22 items, NSR were significantly lower (p < 0.05) when compared to DNSR (yes or yes). In total there was a significant difference between DNSR versus DSR or NSR, respectively, in 19 items. In three items there was no significant difference between DNSR versus DSR or NSR, respectively. The three items with no significant difference were: Cat-confident, PAL-help activities of daily living and PAL-pain-daily-activities. There were no significant difference between DSR and NSR I 6 out of 22 items, the six items being: Cat-Confident, PAL-Felt weak, PAL-Nauseated, PAL-Tired, (PAL-Pain daily activities) and PAL-Tense.
shows that a total of two items CAT ‘Chest felt tight’ and PAL ‘Pain daily activities’ did not show significance in DNSR versus DSR or NSR. Nor did they show improvement in recognition rates DNSR versus DSR or NSR.
Discussion
Acknowledging symptoms is a prerequisite for alleviation and thus palliative care. The main findings of the present study is:
A substantial discrepancy between symptoms of high intensity described by patients with COPD on CAT and EORTC-QLQ-C15-PAL during hospitalization and the rates of which, they are recognized by health professionals in the medical records.
Highly recognized rates were in five out of seven symptoms obtained by mandatory screenings.
Most prevalent symptoms of high intensity can be described as diffuse weakness.
Items recognized by a rate< 70% were all symptoms with treatments and thus possible alleviation by palliative care.
A team-based symptom recognition DNSR is superior to DSR or NSR.
In the 14 symptoms out of 44 recognized by a rate > 70%, 7 were recognized by very high rates ranging between 90% and 100%. From the seven very highly recognized rates, five were obtained by mandatory screenings of functionality conducted by the nurses: NSR PAL ‘Short walk outside’ 93%, NSR PAL ‘Bed/chair’ 92%, NSR PAL ‘help activities of daily living’ 100%, NSR PAL ‘Appetite’ 95% and NSR CAT‘ Activities at home’ 94%.
The remaining two rates of very high recognition were doctors’ recognition of the main symptom of patient with COPD being dyspnoea exemplified by: DSR PAL ‘Short of breath’ 97% and DSR CAT ‘breathless’ 100%. The high level of recognition among the doctors of these main symptoms of COPD is expected in a specialized respiratory unit, and is much higher than what was found among doctors (46%) in specialized palliative cading to Strömgren et al [Citation24].
It is striking that these main symptoms of COPD are not recognized by nurses in respiratory units to a degree > 70%. One possible explanation may be that vital values such as saturation, respiratory quince and supplemental oxygen are documented by numbers according to algorithms.
One of 14 items recognized by rates> 70% describes a psychological symptom. This is in concordance with Strömgren et al. proclaiming that physical symptoms much more often are recognized when compared to psychological symptoms [Citation24].
This distribution is concerning as this study was conducted in a population of patients with COPD with literature giving evidence that the main symptom dyspnoea affects patient’s cognitive and affective functions including anxiety and depression [Citation44–Citation46] and that prevalence of anxiety is 10 times higher among patients with COPD versus the average population [Citation47,Citation48]. With 42.5% of included patients claiming to feel tense, corresponding to a severe intensity; an indication of a significant symptom that is recognized in literature but not recognized sufficient in practice is given.
Most patients with COPD indicated that they felt very limited in all activities at home, felt a reduction in their energy level and had a feeling of being very weak and tired. Only the patient’s limitation in activities at home was recognized to a degree> 70% (NSR 94%).
The described symptomatology could be presented as diffuse weakness difficult to intervene on. According to Stömgren et al. medical records often tends to be action-oriented and focuses on symptoms you can intervene on[Citation23,Citation24], which might be part of the explanation of the relatively low rates of recognition.
Patients describing the symptom ‘pain daily activities’ of high intensity were not recognized at all by either doctors or nurses. Complete lack of recognition might be prevented if patient symptomatology is screened in fixed algorithms.
In 30 out of 44 items DSR or NSR were < 70%. Items recognized by a rate< 70% were all symptoms with possible treatments and thus potential to be alleviated by palliative care. The bigger the discrepancy the bigger the risk, those symptoms with potential of alleviation, is neither recognized by doctors nor nurses.
Only two items did neither show an improvement in recognition rates when a team-based recognition was compared to either DSR or NSR, nor did they show a significant difference between recognition rates of either DSR or NSR versus DNSR. Since 19 out of 22 items were improved in recognition rates if DNSR was compared to either DSR or NSR, it is explicit that a team-based recognition is preferable to both DSR and NSR if total symptom recognition is at aim. A method to elucidate symptoms could be by mandatory screenings repeated by fixed algorithms and based upon a team-based intervention to ensure recognition. A procedure could be nurses obtaining PRO-data by relevant questionnaires and doctors contra-signing them.
Ethical considerations as to what resources patients were asked to use on questionnaires was relevant, making it a priority to access data via short schemes in recognition of the patients’ often weakened condition. Despite significant physical barriers, there was great support amongst patients as well as relatives, who often stated that they experienced the problem as meaningful and current, in full compliance with previous studies within palliation[Citation49,Citation50].
When interpreting symptom intensities and recognition rates of this study in relation to palliative care, it is necessary to take into account that seven patients were excluded due to an acute and instable respiratoric state or because they were evaluated to weak for participation, therefore being in an increased risk of dying. Thus exclusion of these patients might bias our results.
Another point to take into consideration is the fact that included patients might not reflect the population that EORTC-QLQ-C15 aims at, being a population with a life expectancy of only a few months. Our included population is however in concordance with WHO’s definition of palliation[Citation16] and reflects the with span of the disease trajectory as well as the acknowledgement that a prediction of life expectancy in COPD is not reliable [Citation51].
EORTC-QLQ-C30 was shorten into EORTC-QLQ-C15-PAL by IRT based on a population n = 8242 of which n = 904 (11%) was characterized as palliative [Citation42]. No evidence of different item function (DIF) was found between ‘palliative patients’ versus ‘non-palliative patients’ and the ‘general population’ [Citation42]. This might provide a psychometric argument to use EORTC-QLQ-C15-PAL on a population with a life expectancy beyond a few months as presented in this study.
In CAT six out of eight items refers to respiratory symptoms reducing the questionnaire’s ability to reflect the multi-morbidity this population is known by.
Limitations
Since CAT and EORTC-QLQ-C15-PAL have been validated on different populations it is not given that measures between the two are equivalent making the two questionnaires able to correspond. Correspondence and equivalence between CAT and EORTC-QLQ-C15-PAL are thus arbitrary and should be interpreted as such.
Furthermore EORTC-QLQ-C15-PAL has not been validated for patients with COPD giving us no basis to calculate statistical power and therefore a limited ability to evaluate the data statistically. Our main focus has been recognition rates and we have no reason to believe that they would change significantly if more patients were included which is why a relatively small sample size n = 40 was accepted for this study as we are pioneering the cross-field between COPD care and palliative care.
Interpretation and generalizability of the data extracted from the medical records may be put to question as both units used the same documentation-system (Epic). Since our main results en general reflect the ones found in earlier studies [Citation23,Citation24] we believe it advocates for the generalizability of our results.
Conclusion
This study illustrates a symptomatology that reaches beyond CAT and into EORTC-QLQ-C15-PAL in a population with COPD showing the most prevalent symptoms with high intensity in the sample can be described as diffuse weakness.
To our knowledge our study is the first to put fourth evidence of a substantial discrepancy between symptoms described by patients with COPD and the degree by which they are recognized by health professionals. Symptoms with low recognition rates all had potential treatments and thus options of alleviation if recognized.
It is shown that a team-based symptom recognition DNSR is superior when compared to DSR or NSR. Team-based systematic screening is suggested as a pathway to increase symptom recognition in patients with COPD.
Current results, discussion and conclusion reveal symptoms identified with PRO-data by a simple procedure that could be the focus of interest in relation to new studies on palliation for patients with COPD. New screening-tools able to reveal a wide range of symptoms elucidating the multi morbidity and complexity of patients with COPD could contribute positively to the development of palliative care for this very population.
Acknowledgments
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Notes on contributors
Charlotte Sandau
Charlotte Sandau is a Clinical Nursing Specialist in the Medical Unit of Hvidovre Hospital in Copenhagen. She has a degree of MSc in Health Science and is a RN with prior clinical experience within respiratory medicine. She has a special interest in non-malign palliatve care.
Dorthe Gaby Bove
Dorthe Bove is a postdoctoral researcher in nursing. She has a wide interest in the psychosocial aspects of COPD and non-malign palliative care, and has a primary focus on self-management strategies.
Kristoffer Marsaa
Kristoffer Marsaa is a MD specialist in respiratory medicine, senior consultant, palliative unit Herlev and Gentofte hospitals. Clinical and research interest in COPD, pulmonary fibrosis, palliative care and consequences of life-threatening disease measured by patient reported outcomes.
Camilla Sørli Bekkelund
Camilla Sørli Bekkelund is a RN working within respiratory medicine with a special interest in non-malign palliative care.
Matias Greve Lindholm
Matias Greve Lindholm is a senior doctor at University Hospital Copenhagen.He is a trained cardiologist with special interest in out of hospital cardiac arrest and cardiogenic shock. He is at the moment working at the intensive cardiac care unit. Besides he is an associate professor at the University of Copenhagen participating in both pre- and postgraduate education.
References
- Pauwels RA, Rabe KF. Burden and clinical features of chronic obstructive pulmonary disease (COPD). Lancet. 2004;364;613–620.
- Murray CJL, Lopez AD. Alternative projections of mortality and disability by cause 1990 – 2020: global burden of disease study.The Lancet. 1997; 349:1498–1504.
- Andreassen H, Vestbo J. Chronic obstructive pulmonary disease as a systemic disease: an epidemiological perspective. Eur. Respir. J. 2003;22(Supplement 46):2s–11.
- Decramer EM, Rossi A, Anto JM. SERIES ‘contributions from the European respiratory monographs’ epidemiology of chronic obstructive pulmonary disease. European Respiratory Journal. 2001;17:982–994.
- “WHO. Chronic obstructive pulmonary disease (COPD) fact sheet,” 2016. [Online]. Available: http://www.who.int/mediacentre/factsheets/fs315/en/.
- “Global Initiative for Chronic Obstructive Lung Disease. Global strategy for diagnosis, management and prevention of COPD,” 2017.
- A. L. A. E. and S. U. R. and H. E. Division, “Trends in COPD (Chronic Bronchitis and Emphysema): Morbidity and Mortality,” 2013.
- Blinderman CD, Homel P, Billings JA, et al. Symptom distress and quality of life in patients with advanced chronic obstructive pulmonary disease. J. Pain Symptom Manage. 2017;38(1):115–123.
- Decramer M, Janssens W, Miravitlles M. Chronic obstructive pulmonary disease. Lancet. 2012;379(9823):1341–1351.
- Miranda DG, María E, Peces S, et al. HOLD study (home care obstructive lung disease): natural history of patients with advanced COPD. BMC Palliat. Care. 2016(15:35);1–9.
- Ns J, Montagnini ML. Rehabilitation of the hospice and palliative care patient. J. Palliat. Med. 2011;14(5):638.
- Janssen DJA, Mccormick JR. Palliative care and pulmonary rehabilitation. Clin. Chest Med. 2017;35(2):411–421.
- Mccormick JR, “Pulmonary Rehabilitation and Palliative Care.”
- Murray SA, Boyd K, Kendall M, et al. “Dying of lung cancer or cardiac failure, : prospective qualitative interview study of patients and their carers in the community,”. BMJ. 2001;325(325):929.
- Murray SA, Kendall M, Boyd K, et al. Clinical review Illness trajectories and palliative care. BMJ. 2005;330:1007–1011.
- “WHO | WHO Definition of Palliative Care.” [Online]. Available: http://www.who.int/cancer/palliative/definition/en/.
- Bausewein. Understanding breathlessness: cross-sectional comparison of symptom burden and palliative care needs in chronic obstructive pulmonary disease and cancer. J. Palliat Med. 2010;sep13(9):1109–1118.
- Lilly EJ, Senderovich H. Palliative care in chronic obstructive pulmonary disease. J. Crit. Care. 2017;35(2016):150–154.
- Al WE, Dj J, Ma S, et al. Symptoms, comorbidities, and health care in advanced chronic obstructive pulmonary disease or chronic heart failure. J Palliat Med. 2011;14(6):735–743.
- Goodridge. Health care utilization of patients with chronic obstructive pulmonal disease and lung cancer in the last 12 months of life. Respir Med. 2008;june 102(6):885–891.
- Hardin KA, Mayers F, Louie S. Integrating palliative care in severe chronic obstructive lung disease COPD. COPD. 2008;Aug(5):207–220.
- Johsi M, Johsi A, Bartter T. Symptom burden in chronic obstructive pulmonary disease and cancer. Curr Opin Pulm Med. 2012;Mar(18):97–103.
- Strömgren AS, Groenvold M, Soerensen A. Symptom recognition in advanced cancer. A comparison of nursing records against patient self-rating. ACTA Anaesthesiologica Scandinavica. 2001;45:1080–1085.
- Strömgren AS, Groenvold M. Does the medical record cover the symptoms experienced by cancer patients receiving palliative care? A comparison of the record and patient self-rating. Journal of Pain and Symptom Management. 2001;21(3):189–196.
- Ng KVGC. Symptoms and attitudes of 100 consecutive patients admitted to an acute hospice/palliative care unit. J Pain Symptom Manag. 1998;16:307–316.
- As S, Goldschmidt D, Groenvold M, et al. Self-assesment in cancer patients reffered to palliative care: a study of feasibility and symptom epidemiology. Cancer. 2002;94:512–520.
- Schuit MB, Sleijfer KW, Meijer DT, et al. FCM, “Symptoms and functional status of patients with dissiminated cancer visiting outpatient departments,”. J Pain Symptom Manag. 1998;16:290–297.
- Jones Poul JC, Otto B, “COPD assessment Test CAT,” 2009.
- EORCT-GROUP, “EORTC QLQ-C15-PAL (version 1).”
- Jones PW, Harding G, Berry P, et al. Development and first validation of the COPD assessment test. European Respiratory Journal. 2009;34(3):648–654.
- Jones Poul BO, Christine J. COPD Assesment Test CAT -PROFESSIONAL Expert guidance on frequently asked questions introducing the COPD Assessment Test TM (CAT). Healthcare Professional User Guide. 2012;3:1–15.
- Pw J, Bruselle G, Dal NRW. Properties of the COPD assessment test in a cross-sectional European study. EUR Respir J. 2011;38:29–35.
- Jones PW, Tabberer M, Chen W. Creating scenarios of the impact of COPD and their relationship to COPD assessment test (CAT TM) scores. BMC Pulm. Med. 2011;11(1):42.
- EORTC-group EORTC QLQ-C30. .
- Groenvold M, Dewolf L. Addendum to the EORTC QLQ-C30 scoring manual : scoring of the EORTC QLQ-C15-PAL. 2006;July:1–10.
- Mclachlan S, Devins GM, Goodwin PJ. Original paper validation of the European organization for research and treatment of cancer quality of life Questionnaire (QLQ-C30) as a measure of psychosocial function in breast cancer patients. European Journal of Cancer. 1998;34(4):510–517.
- Ringdal G, Ringdal K. Testing the EORTC quality of life questionnaire on cancer patients with heterogeneous diagnoses linked references are available on JSTOR for this article: testing the EORTC quality of life questionnaire on cancer patients with heterogeneous diagnoses. Quality of life Research. 2017;2(2):129–140.
- Groenvold M, Carol M, Spungers MAG, et al. Validation of the EORTC QLQC30 quality of life questionnaire through combined qualitative and quantitative assessment of patient-observer agreement.J Clin Epidemiol. 1997;50(4):441–450.
- EORTC-group. EORTC QLQ-C30 scoring manual the eortc qlq-c30 introduction. 2001;30.
- King AMT. The interpretation of scores from the eortc quality of life questionnaire QLQ-C30 the interpretation of scores from the EORTC quality of life questionnaire QLQ-C30. Quality of Life Research. 2017;5(6):555–567.
- Groenvold M, Aa M, Aaronson NK, et al. The development of the EORTC QLQ-C15-PAL: a shortened questionnaire for cancer patients in palliative care. European Journal of Cancer. 42 2006;42:55–64.
- Bjorner JB, Petersen MA, Groenvold M, et al. Use of item response theory to develop a shortened version of the EORTC QLQ-C30emotionalfunctioningscale. Quality of Life Research2004;13:1683–1697.
- Aa M, Groenvold M, Aaronson N, et al. Item response theory was used to shorten EORTC QLQ-C30 scales for use in palliative care. Journal of Clinical Epidemiology 2006;59:36–44.
- Carlucci S, Guerrieri A, Nava A. Palliative care in COPD patients: is it only an end-of-life issue?. Eur. Respir. Rev. 2012;21(126):347–354.
- Janssens T, De Peuter S, Stans L, et al. Dyspnea perception in COPD: association between anxiety, dyspnea-related fear, and dyspnea in a pulmonary rehabilitation program. Chest. 2011;140(3):618–625.
- Tetikkurt C, Ozdemir I, Tetikkurt S, et al. Anxiety and depression in COPD patients and correlation with sputum and BAL cytology. Multidiscip Respir Med. 2011;6(4):226–231.
- Livermore N, Sharpe L, McKenzie D. Panic attacks and panic disorder in chronic obstructive pulmonary disease: a cognitive behavioral perspective. Respir. Med. 2010;104(9):1246–1253.
- Heslop K. Non-pharmacological treatment of anxiety and depression in COPD. Nurse Prescr. 2014;12(1):43–47.
- White C, Hardy J. What do palliative care patients and their relatives think about research in palliative care – a systematic review. Support Care Cancer.. 2010;2010(Aug):1.
- Gysels M, Shipman C, Hons BA. I will do it if it will help others :”’ motivations among patients taking part in qualitative studies in palliative care.Journal of Pain and Symptom Management 2008:35(4)347–355.
- Mittal R, Chhabra SK, Mittal R, et al. GOLD classification of COPD : discordance in criteria for symptoms and exacerbation risk assessment gold classification of COPD : discordance in criteria for symptoms and exacerbation risk assessment. COPD Journal of Chronic Obstructive Pulmonary Disease 2017:2555(February):14(1): 1–6.