ABSTRACT
Background: New, complex, and expensive therapies targeting Interleukin-5 (IL-5) to treat severe eosinophilic asthma are emerging.
Objective: To assess efficacy, adverse events, and inter-drug comparison of mepolizumab and reslizumab for treating severe eosinophilic asthma.
Design: A systematic review and meta-analysis on randomized, placebo-controlled, clinical trials elucidating two critical (exacerbation rate and oral corticosteroid (OCS) use) and six important clinical outcomes on the efficacy and safety of mepolizumab and reslizumab.
Results: Five studies (N = 2197) contributed with data for exacerbation rate, showing a reduction of 53% (95% CI 46; 59) in favour of anti-IL-5, corresponding to –0.94 annual exacerbations (95% CI –1.08;–0.82), thus exceeding the predefined minimal clinical important difference (MCID) of 25% reduction of the estimated ≥2 annual exacerbations. Quality of evidence was considered moderate, with low heterogeneity in study findings (I2 = 0%). One study (N = 135) contributed with data on percentage of patients experiencing ≥50% reduction inoral corticosteroid treatment, showing an effect of 20% (95% CI 2.3;47) in favour of anti-IL-5 treatment (mepolizumab), thus exceeding the predefined MCID of 10%. Quality of evidence was considered low.
Compared to placebo, anti-IL-5 showed significant improvements in lung function, asthma control, and asthma-related quality of life, but below the MCIDs. No differences were observed for serious adverse events and number of patients, who dropped out. No studies evaluating sickleave or head-to-head comparisons were identified. By indirect comparison, we found no significant difference between mepolizumab and reslizumab in any ofthe predefined clinical outcomes. OCS treatment reduction could not be compared due to lack of reslizumab studies investigating this outcome.
Conclusions: Mepolizumab and reslizumab provide significant and clinically relevant improvements in exacerbation rate and OCS reduction. Indirect, inter-study comparisons revealed no differences between the anti-IL-5 drugs in efficacy or safety measures.
Background
Asthma is a common chronic inflammatory airway disorder affecting 300 million people worldwide [Citation1]. Severe asthma is defined by frequent exacerbations despite treatment with high-dose inhaled corticosteroids (ICS) plus a second controller, or the need for daily oral corticosteroid treatment (OCS), to prevent exacerbations and achieve proper asthma control or stay uncontrolled on this treatment [Citation2,Citation3]. The prevalence of severe asthma is estimated 3–15% of all patients with asthma, depending on the method of identification [Citation4] and is associated with a significant negative impact on quality of life for the affected patients [Citation5], as well as an increased risk of morbidity and mortality [Citation6]. The uncontrolled severe asthma patient is a smaller part of the severe asthma patients, estimated one-third of all severe asthma patients [Citation7].
Asthma has traditionally been categorised as either allergic- or non-allergic. However, it is now increasingly recognised that asthma is a heterogeneous syndrome consisting of several immunological subtypes with differing disease mechanisms, which has opened an avenue for personalised pharmacological treatment targeting these endotypes [Citation8]. A well-defined disease endotype is eosinophilic asthma, where an increased concentration of eosinophil granulocytes in the blood and bronchial mucosa leads to release of pro-inflammatory mediators, causing bronchial hyperresponsiveness and poorly controlled asthma [Citation8]. The first specific treatment to target eosinophil asthma was mepolizumab, marketed in Europe in 2015. Subsequently, reslizumab was approved and marketed in 2016.
Both treatments are humanised IgG monoclonal antibodies with a high affinity for interleukin-5 (IL-5), neutralising IL-5 by binding to epitopes on the IL-5-Rα-binding domain [Citation9]. Both mepolizumab and reslizumab have picomolar affinity in inhibiting IL-5 activity, which practically neutralises the molecular target [Citation10].
Both treatments have shown beneficial effects in randomised controlled trials of severe eosinophilic asthma with the potential to halve the rate of asthma exacerbations. However, a more broad evaluation of the clinical benefits as well as a comparison of the two anti-IL-5 treatments is lacking.
Therefore, in spring 2016 the Danish Medicines Council initiated this systematic review and meta-analysis on the efficacy and adverse events of the two anti-IL-5 medicines, mepolizumab and reslizumab, in order to assess the evidence of clinical effects and to assess whether one anti-IL-5 treatment was superior to the other for treating severe uncontrolled eosinophilic asthma.
Methods
The Danish Medicines Council appointed an Expert Committee, which included clinicians in respiratory diseases, paediatrics, and clinical pharmacology, as well as clinical pharmacists, and patient representatives. Assisted by the Danish Medicines Council Secretariat, the Expert Committee initially developed a protocol defining the clinical questions, structured as PICO (Patient, Intervention, Comparator, Outcome) questions and defined the minimal clinically important differences between treatment and placebo or between the two anti-IL-5 medicines [Citation11]. The Secretariat aided with the literature search, selection of relevant papers, data-extraction, and data analysis.
Protocol and registration
This systematic review and meta-analysis is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement [Citation12]. The protocol was developed before the literature search was conducted following the Danish Medicines Councils method handbook [Citation13]. The protocol and final report have been published in Danish on the Danish Medicines Councils website [Citation14,Citation15].
Eligibility criteria
We conducted a literature search involving all studies of adults (≥18 years) with asthma treated with mepolizumab or reslizumab, to answer the predefined PICO questions:
(1) Which adult patients with severe eosinophilic asthma should be offered anti-IL-5 therapy?
Population – Patients ≥18 years of age with severe, eosinophilic asthma. The patient population was not specified further, because the results from the literature search were anticipated to answer which patient characteristics would qualify for anti-IL-5 therapy.
Intervention – Anti-IL-5 therapy (reslizumab 3 mg/kg intravenous administration every 4 weeks, or mepolizumab fixed dose 100 mg subcutaneous administration every 4 weeks) on top of standard care.
Comparator – Placebo on top of standard care.
Outcome(s) (minimal clinical important difference [MCID]) – Of critical importance: excacerbation rate (a reduction in annual rate of at least 25%, corresponding to a minimum reduction of 0.5 excerbations per year); OCS (1) average %-reduction in daily dose [maintenance-treatment] (at least 20% and at least 2.5-mg prednisolone-equivalent dose), (2) percentage of patients who discontinued OCS (a minimum of 5%-points, (3) percentage of patients who achieve a ≥50% reduction of OCS dose (a minimum of 10%-points). Please refer to for definitions of outcomes considered important, and less important.
(2) Is there clinically relevant difference between mepolizumab and reslizumab in the treatment of patients with severe, eosinophilic asthma?
Population – Patients ≥18 years with severe, eosinophilic asthma.
Intervention – Reslizumab 3 mg/kg intravenous administration every 4 week on top of standard care.
Comparator – Mepolizumab fixed dose of 100-mg subcutaneous administration every 4 weeks on top of standard care.
Outcome(s) (MCID) – of critical importance: excacerbation rate (a reduction in annual rate of at least 25%, corresponding to a minimum reduction of 0.5 excerbations per year); OCS (1) average %-reduction in daily dose [maintenance-treatment] (at least 20% and at least 2.5-mg prednisolone-equivalent dose), (2) percentage of patients who discontinued OCS (a minimum of 5%-points, (3) percentage of patients who achieve a ≥50% reduction in OCS dose (a minimum of 10%-points) ().
Table 1. List of outcome measures. For each outcome measure, the importance is indicated, and for critical and important outcome measure the minimal clinically important difference is reported.
The approved dose of mepolizumab is 100 mg administered subcutaneously, whereas some studies examined 75-mg intravenous injections. However, since 100-mg subcutaneous and 75-mg intraveneous mepolizumab are considered equipotent doses, we included studies of both. We excluded studies examining other doses than the above as they have not been approved as standard therapy in the EU, according to mepolizumab’s summary of products characteristics [Citation16]. We used the Danish Society of Respiratory Medicines definition, evaluation, and treatment of severe asthma, which is based on the international ERS/ATS guidelines [Citation2,Citation17].
Information sources
To identify eligible systematic reviews and randomised controlled trials, we searched MEDLINE, Embase, and relevant databases from the Cochrane Library (see Supplementary Appendix Table 1 for detailed search strings). In MEDLINE, all available databases were searched so that records not yet MEDLINE-indexed were identified. In the Cochrane Library, the databases Cochrane Database of Systematic Reviews (CDSR), Cochrane Central Register of Controlled Trials (CENTRAL), and Database of Abstracts of Reviews of Effects (DARE) were searched.
We also searched the following databases to identify relevant clinical guidelines: National Guidelines Clearinghouse, Guidelines International Network, National Institute for Health and Care Excellence, Cochrane Library Technology Assessments (HTA), and the Danish Society of Respiratory Medicine. Market authorisation holders and members of the Expert Committee could also contribute with relevant literature.
Search
Searching MEDLINE and Embase, we identified systematic reviews using the built-in ‘Systematic review’ filter. For identification of randomised controlled trials, a customised filter with high sensitivity was used. No publication language or date limits were applied. The search strategy was developed based on input from all Expert Committee members by an information specialist from the Danish Medicines Council secretariat (see Supplementary Appendix Table 1 for the complete search strings with annotations). The searches were conducted on 15 June 2017. Initially, the search strategy was designed also to include identification of records solely mentioning omalizumab. However, according to the eligibility criteria only records mentioning reslizumab and/or mepolizumab were considered possibly relevant.
Manual screening of reference lists of the identified studies was carried out by at least two members of the Expert Committee and the secretariat. References not already identified in the initial search were screened by full text reading.
Study selection
Two persons from the Danish Medicines Council’s secretariat independently screened the identified guidelines and assessed whether they were relevant to answer the PICO questions. The same two independently screened the identified systematic reviews and randomised controlled trials on title and abstract level. Next, they independently screened the selected systematic reviews on full-text level and assessed whether they met the predefined criteria for inclusion. The selected randomised controlled trials were screened on full-text level by one of the aforementioned two and at least one Expert Committee member. Any disagreements were resolved by consensus-based discussion.
Data collection process and data items
After the study selection process was finalised, all relevant data were extracted by one person and validated by another. Bibliographical and study description data, patient characteristics, and data related to the primary and secondary outcomes were extracted from the included studies.
Before the search and data extraction, the outcomes were classified as ‘critical’, ‘important’, and ‘less important’ depending on the clinical relevance of the outcome measure. By consensus by the Expert Committee members, measure units were identified, as well as the MCIDs. MCIDs were chosen based on previously validated thresholds, where possible [Citation18].
Predefined subgroup analysis included sex, age, age at asthma onset, blood eosinophil count, nasal polyposis present, inhalation allergy present, and treatment intensity according to Global Initiative for Asthma (GINA) (in order to assess the criteria for initiating anti-IL-5 therapy) [Citation1]. The subgroup analyses were not included in the meta-analysis, but were described narratively.
Risk of bias in individual studies
Two persons from the Danish Medicines Council’s secretariat independently assessed the methodological quality of eligible studies using the Cochrane collaboration’s tool for assessing risk of bias. The assessment included evaluation of risk of bias across the following six domains: sequence generation, allocation concealment, blinding (of participants, personnel, and outcome assessors), incomplete outcome data, selective outcome reporting, and other sources of bias. The final judgement was categorised into low, high, or unclear risk of bias [Citation19].
Summary measures and synthesis of results
The evidence of each clinical question was examined and is presented per outcome measure. We used intention-to-treat analyses with hazard ratios (HR), relative risks, rate ratios, or odds ratios (OR) for dichotomous outcome measures. For continuous outcome measures, we used mean difference (MD) or standardised mean difference (SMD). We did not impute missing data.
The meta-analyses were conducted with the inverse variance method with the assumption of random effects. For indirect comparisons of the effect of mepolizumab vs. reslizumb, Bucher’s method for adjusted indirect comparison was used [Citation20]. Statistical test for heterogeneity was performed (Cochran’s Q) and degree of heterogeneity was described with the I2 statistic.
Confidence in cumulative evidence
We used the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach to assess the quality of evidence provided by the meta-analyses [Citation21]. The following domains were evaluated: study design, risk of bias, inconsistency, indirectness, imprecision, and other considerations. The secretariat conducted the systematic GRADE-based assessment of the literature, with input from the Expert Committee. Depending on the basic design of the included studies and any subsequent downgrading, the evidence quality was categorised as high, moderate, low, or very low.
Results
Study selection
Guidelines and systematic reviews
A total of 2 clinical guidelines and 10 systematic reviews with potential relevance of answering the predefined PICO questions were identified (see Supplementary Appendix Table 2). None of these had direct transferability to answer the PICO questions, and were not included for further analysis.
Primary literature
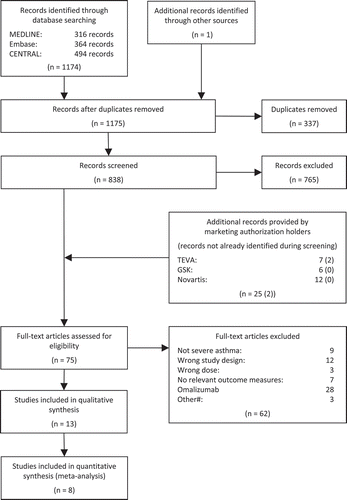
The systematic literature search identified four randomised controlled trials which examined the efficacy of mepolizumab [Citation22–Citation25], and five trials (published in four papers) which examined the efficacy of reslizumab [Citation26–Citation28]. All studies had placebo as comparator. The paper by Castro et al. described two duplicate randomised controlled trials [Citation29]. In the subsequent paragraphs, we have named study 1 Castro 2015a, and study 2 Castro 2015b. The study by Bel et al. [Citation25] was not included in the meta-analysis because the study design differed significantly from the other studies. The results from Bel et al. were instead described for each outcome.
We received 25 papers from the marketing authorisation holders. Twenty-three studies were already identified, one study was excluded due to the wrong study design, and one study that was published after the literature search was conducted was assessed on full-text level as it contained relevant subgroup analyses.
Five studies that included relevant subgroup analysis were identified, three regarding mepolizumab [Citation30–Citation32], and two regarding reslizumab [Citation33,Citation34]. In total, we included 13 studies for further analysis, and eight of those were included in the meta-analysis (see literature selection flow chart in ).
Study characteristics
The study characteristics varied significantly between the included studies, especially in regard to design (randomised double-blind, placebo-controlled trials with single- or multiple phases; randomised double-blind double-dummy placebo-controlled studies), follow-up length (range from 15 to 52 weeks), intensity of the standard of care asthma therapy (all mepolizumab studies included patients with a treatment-intensity equalling severe asthma whereas the majority of all reslizumab studies included patients with a treatment intensity equalling moderate to severe asthma), eosinophil count at treatment initiation (mepolizumab: 150–300 eosinophils per microL depending on the time of measurement; the majority of reslizumab studies included patients with ≥400 eosinophils per microL, and one study included patients with ≥3% sputum eosinophils), and number of previous exacerbations (from no previous exacerbations to a minimum of two). Heterogeneity in results was examined in terms of difference in design and characteristics. An overview of the included studies is presented in the Supplementary Appendix Tables 3 and 4.
Synthesis of results
Exacerbations
Average reduction in the annual number of exacerbations
Combined
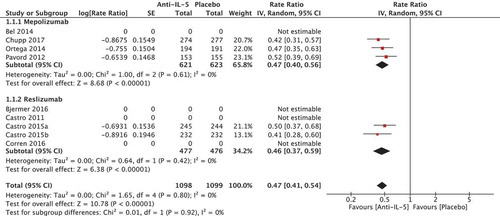
In total, five randomised trials reported in four papers [Citation22–Citation24,Citation29] comprising a total of 2197 patients were included in the meta-analysis. The rate ratio for the number of annual exacerbations showed a favourable effect in the anti-IL-5 group compared to placebo (rate ratio 0.47 [95% CI 0.41; 0.54), which can be translated into an absolute risk reduction of 53% (95% CI 46; 59) (). The absolute annual effect on exacerbations was −0.94 yearly exacerbations (95% CI −1.08; −0.82) in favour of the anti-IL-5 group compared to placebo, calculated based on an estimate of the annual exacerbation rate in Danish patients eligible for anti-IL-5 treatment of minimum of two exacerbations per year (). This effect was larger than the predefined MCID of 25% (a minimum reduction of 0.5 exacerbations per year). The heterogeneity was low (I2 = 0%, p = 0.80) and quality of evidence was considered moderate.
Table 2. Summary of main results.
Mepolizumab
Three studies were included comprising 1244 patients [Citation22–Citation24]. The rate ratio of annual exacerbations was 0.47 [95% CI 0.40; 0.56] in favour of the mepolizumab group compared to placebo. The heterogeneity was low (I2 = 0%).
Reslizumab
Two RCTs reported in the same paper were included comprising 953 patients [Citation29]. The rate ratio of annual exacerbations was 0.46 [95% CI 0.37; 0.59] in favour of the reslizumab group compared to placebo. The heterogeneity was low (I2 = 0%).
Number of patients who experience 0 exacerbations annually
Combined
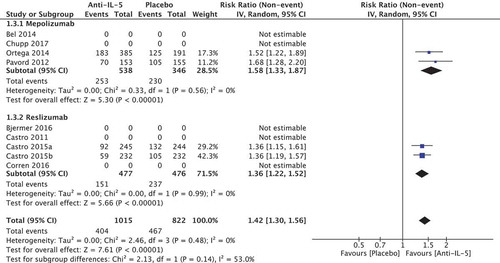
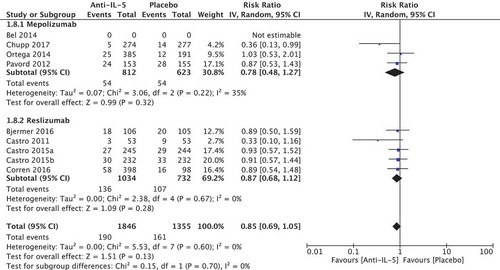
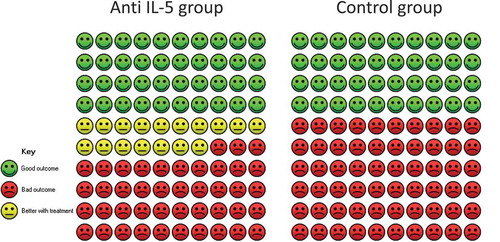
In total, four randomised trials reported in three papers comprising a total of 1837 patients were included in the meta-analysis. We found a relative improvement of 1.42 (95% CI 1.3; 1.56) on the percentage of patients experiencing 0 exacerbations in favour of the anti-IL-5 group (). The calculated absolute difference was 16.9% (95% CI 12.1; 22.5) compared to placebo, which can be translated to 40 out of 100 who experience 0 exacerbations in the placebo group compared to 57 out of 100 in the anti-IL-5 group (). This was larger than the predefined MCID of 10 percentage points. The heterogeneity was low (I2 = 0%, p = 0.48) and quality of evidence was considered moderate.
Figure 4. Cates plot illustrating percentage of patients who experience 0 exacerbations. The Cates plot is based on the absolute effect calculated by the median for the control non-event rate in the included studies (40.2%). This gives a difference of 16.9%-points in the percentage of patients who experience 0 exacerbations.
Mepolizumab
Two studies were included comprising 884 patients [Citation22,Citation24,Citation25]. The relative improvement was 1.58 (95% CI 1.33; 1.87) on the percentage of patients experiencing 0 exacerbations in favour of the anti-IL-5 group. The heterogeneity was considered low (I2 = 0, p = 0.56).
Reslizumab
Two RCTs reported in the same paper were included comprising 953 patients [Citation29]. The relative improvement was 1.36 (95% CI 1.22; 1.52) on the percentage of patients experiencing 0 exacerbations in favour of the anti-IL-5 group. The heterogeneity was considered low (I2 = 0, p = 0.99).
The subgroup analyses showed that the degree of blood eosinophilia, number of previous exacerbations, and the intensity of underlying asthma treatment could have an influence on the effect of the anti-IL-5 treatment. Subgroup analyses show that the effect of anti-IL-5 treatment is more pronounced in late-onset asthma compared to early-onset (see Supplementary Appendix – subgroup analysis, narrative overview for details).
Oral corticosteroid (OCS) treatment
Median reduction and percentage of patients who experienced ≥50% reduction of OCS
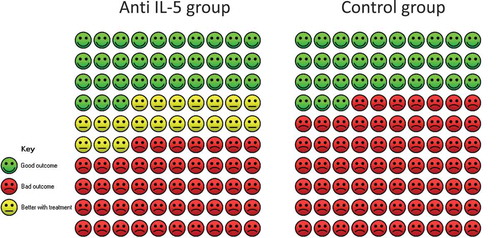
A single randomised study (n = 135) of mepolizumab was included for further analysis [Citation25], which showed a median reduction of OCS of 50% (95% CI 20; 75) compared to a 0% (95% CI −20; 33.3) reduction in the placebo group. Due to the lack of statistical evaluation of the average reduction in OCS between mepolizumab and placebo, it was not possible to assess the predefined MCID of 20%. Instead, we assessed the percentage of patients, who experienced ≥50% reduction in OCS treatment. The relative difference was 1.61 (95% CI 1.07; 2.41) in favour of mepolizumab (22/66 in the placebo group experienced a ≥50% reduction in OCS compared to 37/69 in the mepolizumab group) (). We calculated an absolute effect of 20.3%-points (95% CI 2.3; 47.0), which was larger than the defined MCID of 10 percentage points. The quality of evidence was considered low.
Figure 5. Cates plot of patients who experienced ≥50% reduction in oral corticosteroid treatment. The Cates plot is based on the absolute effect calculated by the median for the control non-event rate in the included studies (33%). This gives a difference of 20.3%-points in the percentage of patients who experience ≥50% reduction in oral corticosteroid treatment.
Percentage of patients who were discontinued OCS
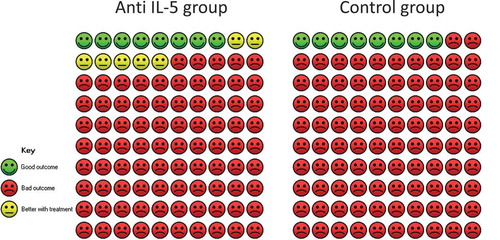
In the mepolizumab group, 10 out of 69 patients were discontinued OCS, whereas 5 out of 66 were discontinued OCS in the placebo group, which accounted for a relative difference of 1.91 (95% CI 0.69; 5.30) in favour of the mepolizumab group. This yielded a 6.9% (95% CI −2.3; 32.6%) in favour of mepolizumab (). The quality of evidence was considered low. We found no studies on the reduction in OCS when using reslizumab. The quality of evidence was considered low.
Figure 6. Cates plot of patients who discontinued oral corticosteroid-maintenance treatment. The Cates plot is based on the absolute effect calculated by the median for the control non-event rate in the included studies (7.6%). This gives a difference of 6.9%-points in the percentage of patients who discontinue oral corticosteroid-maintenance treatment.
Lung function
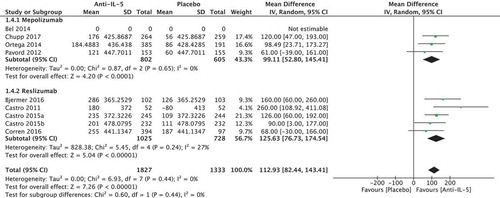
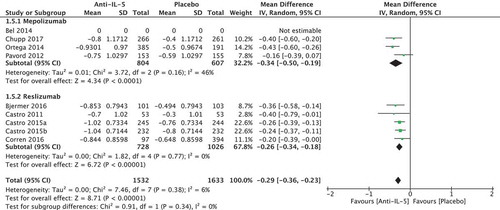
Nine randomised trials of 3160 patients were included in the meta-analysis (four regarding mepolizumab [Citation22–Citation25], and five regarding reslizumab [Citation26–Citation29]). No studies presented the number of patients experiencing the MCID of 200 mL in forced expiratory volume (FEV1). We found an absolute difference of FEV1 of 112.93 ml (95% CI 82.44; 143.31) in favour of the anti-IL-5 treatment compared to placebo (), which is below the minimal clinically important difference. We found no significant heterogeneity (I2 = 0%, p = 0.44). The quality of evidence was considered moderate.
Asthma control
Nine studies of 3165 patients were included; four mepolizumab studies (three using Asthma Control Questionnaire [ACQ]5 [Citation22,Citation23,Citation25], and one ACQ6 [Citation24]), and reslizumab studies (all used ACQ7 [Citation26–Citation29]). We pooled the results from the different ACQ versions in the meta-analysis and found a change of −0.29 points (95% CI −036; −0.23) in the anti-IL-5 group compared to placebo, which was below the minimal clinically important effect of 0.5 points (). No significant heterogeneity was observed (I2 = 6%, p = 0.38). The quality of evidence was considered low.
Quality of life
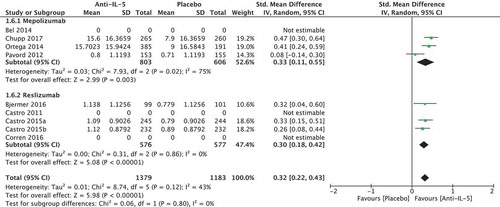
We included four studies using the Asthma Quality of Life Questionnaire (AQLQ): one mepolizumab study [Citation24] and three reslizumab studies [Citation26,Citation29]. Further three studies were included, which used SGRQ, in which the MCID is 4 points [Citation22,Citation23,Citation25], which gave a total of 2562 included patients. We pooled all the results by recalculating the scores to SMD and found a significant improvement of quality of life among patients in the anti-IL-5 group compared to the placebo group (SMD 0.32 [95% CI 0.22; 0.43). We thereafter back-transformed the SMD to AQLQ points by assuming a SD of 1 (the SD was observed to be 0.88–1.12), which showed an improvement of 0.32 (95% CI 0.22; 0.43) in the anti-IL-5 group compared to placebo () that was below the MCID of a 0.5 point improvement. Moderate heterogeneity (I2 = 43%) was observed, but this was not significant (p = 0.12). The quality of evidence was considered low.
Dropout rate
We included nine studies of 3201 patients (four regarding mepolizumab [Citation22–Citation25], and five regarding reslizumab [Citation26–Citation29]), and found a larger dropout rate in the placebo group compared to the anti-IL-5 group (relative risk reduction of 0.85 [95% CI 0.69; 1.05]). Recalculated to absolute values, we found −2.3%-point (95% CI −4.7;-0.7) difference in dropout in the anti-IL-5 group compared to the placebo group (), which was below the MCID of 10%. We found no significant heterogeneity (I2 = 0%, p = 0.28). The quality of evidence was considered moderate.
Serious adverse events (SAE)
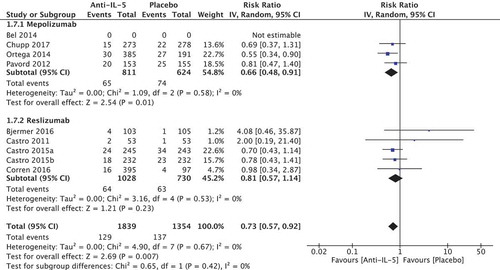
We included nine studies of 3193 patients (four regarding mepolizumab [Citation22–Citation25], and five regarding reslizumab [Citation26–Citation29]), and found an increased risk of SAE in the placebo group compared to the anti-IL-5 group with a relative risk reduction of 0.73 [95% CI 0.57; 0.92] in favour of the anti-IL-5 group. This was recalculated to an absolute value of −2.4%-points (95% CI −0.7; −3.8) (). The effect estimate was not greater than that MCID of ±5% points. The effect was positive for anti-IL-5 treatment and therefore it did not imply a negative impact on the assessment of the medicines. We found no significant heterogeneity (I2 = 0%, p = 0.67). The quality of evidence was considered moderate.
Sick leave
We found no studies evaluating the effect of anti-IL-5 treatment on sick leave.
Risk of bias evaluation
In general, the risk of bias assessed by the Cochrane Risk of Bias Tool was considered low: two studies did not account for random sequence generation or allocation concealment [Citation26,Citation28] and one study did not account for blinding of outcome assessment [Citation24]. A detailed assessment of the quality of evidence (GRADE) is presented in the Supplementary Appendix Table 5.
Comparison of the effect of mepolizumab and reslizumab
Using Bucher’s method of indirect comparison between two effects, we found no significant difference between mepolizumab and reslizumab in any of the predefined clinical outcomes ( and ).
Table 3. The effect of mepolizumab and reslizumab – continuate endpoints.
Table 4. The effect of mepolizumab and reslizumab – dichotome endpoints.
It was not possible to compare the effect on reduction in OCS usage because no studies on reslizumab were published before conducting the bibliographic search. For a detailed overview of the quality of evidence, please refer to Supplementary Appendix Table 6.
Discussion
Summary of the evidence
In this systematic review and meta-analysis of the efficacy and adverse events of the two direct anti-IL-5 treatments, mepolizumab and reslizumab, we found no clinically relevant inter-drug differences in efficacy for any of the 8 predefined critical and important clinical outcomes.
Compared to placebo, both mepolizumab and reslizumab had statistically significant and clinically relevant impact on the exacerbation rate in the meta-analysis. The effect compared to placebo on the reduction in annual exacerbations was pronounced with a 53% absolute risk reduction. In addition, the absolute difference in patients experiencing 0 exacerbations was 16.9 percentage points with 40 out of 100 who experienced 0 exacerbations in the placebo group compared to 57 out of 100 in the anti-IL-5 group. Mepolizumab further showed a clinically relevant reduction in OCS maintenance doses compared to placebo with 20.3 percentage points more in the mepolizumab group being able to halve their maintenance OCS dose, but evidence was not available for reslizumab on that outcome. For both anti-IL-5 drugs, the meta-analysis showed statistically significant effects on lung function, asthma control, asthma-related quality of life, and occurrence of SAEs, but with differences below the prespecified MCIDs.
Generally, the quality of evidence was very low concerning our primary aim of studying inter-drug differences, as only indirect comparison was available due to lack of published studies comparing the drugs head-to-head. The quality of evidence was low to moderate regarding the comparisons of mepolizumab and reslizumab with placebo, and with a low risk of bias.
Our results align with a recent Cochrane review by Farne et al., which also included the newly approved IL-5-receptor antagonist benralizumab, showing a reduction in asthma exacerbations of almost one half in the anti-IL-5 group compared to placebo [Citation35]. Like our meta-analysis, Farne et al. also observed a modest improvement in quality of life and a small improvement in lung function in both mepolizumab- and reslizumab-treated patients. The clinical outcomes in our study were comparable to the outcomes investigated by Farne et al., except that we included OCS maintenance dose reduction and discontinuation as critical outcomes, because the risk of serious adverse events are substantial with such treatment regimen [Citation36]. Importantly, the study by Farne et al., although largely including the same studies, does not provide an inter-drug comparison of efficacy and safety.
Inter-drug comparison
We did not identify any head-to-head studies of reslizumab vs. mepolizumab, but using indirect comparisons we observed comparable effects for all of the predefined critical and important outcomes. This was affirmed by the fact that heterogeneity for the critical and important outcomes in the combined analyses of mepolizumab vs. placebo and reslizumab vs. placebo was deemed low. However, the evidence is considered low with respect to the critical outcome measures, due to the indirect comparison and the lack of studies of reslizumab on reduction in OCS maintenance treatment. Still, given that the pharmacological profiles of the two medicines are comparable [Citation10], we would expect reslizumab to have a beneficial effect on reduction in OCS treatment comparable to that observed for mepolizumab. It is important to emphasise that no studies have been published on this topic yet, and the derived conclusion are based on low-grade evidence.
Inter-study differences
Key points of asthma severity and cut-off for blood eosinophil level differed between studies on the two anti-IL-5 drugs. The majority of the reslizumab studies included patients with moderate asthma, where the use of a second controller was not mandatory [Citation26,Citation28,Citation29]. This is against current asthma treatment guidelines, where the systematic assessment in the GINA criteria clearly states that anti-IL-5 treatment should be used only in patients with severe asthma (GINA step 4/5). However, subgroup analyses showed that among patients receiving ICS plus a long-acting b2-agonist, the effect of reslizumab on exacerbations was comparable to all the included patients, e.g. patients receiving ICS with or without a long-acting b2-agonist.
The blood eosinophil count threshold for inclusion was higher in the reslizumab studies compared to the mepolizumab studies. The majority of reslizumab studies included patients with ≥400 eosinophils per microL, and one study included patients with ≥ 3% sputum eosinophils, whereas the mepolizumab studies included patients with ≥150–300 eosinophils per microL depending on the time of measurement. This could indicate that the reslizumab-treated patients suffered from a more eosinophil-driven disease. However, the number of previous exacerbations was higher in the mepolizumab vs. reslizumab studies, which is a predictor of a good response to anti-IL-5 treatment.
Limitations
Assessment of differences in efficacy and adverse events of mepolizumab and reslizumab was only possible using indirect comparison by Bucher’s method for aggregated data as no head-to-head studies were available. This method is prone to produce more uncertain results due to the difference in inclusion criteria and design of the different studies.
It is a limitation that only data on mepolizumab and only from one study were available with reduction in daily OCS use as primary outcome [Citation25]. Furthermore, that study did not provide data on the predefined critical outcome ‘Percentage of patients who discontinued OCS’. Due to this, the Expert Committee defined the outcome measure ‘Percentage of patients who achieve a ≥50% reduction in OCS dose’ after the protocol had been approved by the Danish Medicines Council.
An important limitation not discussed in previous reviews of anti-IL-5 treatment is the external validity. The drugs are expensive, and at present, initiation of anti-IL-5 treatment should not be considered for patients not optimally treated according to GINA or other recognised international asthma guidelines. Unfortunately, clinical assessment prior to inclusion is not described in detail in any of the available studies. This is of utmost importance as it is now widely acknowledged that systematic assessment of asthma phenotype, triggers, comorbidities, and obstacles to asthma control to differentiate between difficult-to-treat asthma and severe asthma is needed to increase asthma control and prevent initiation of irrelevant expensive treatments [Citation37–Citation39]. In England, the National Health Service (NHS) has established specialised respiratory services to centralise the systematic assessment of difficult-to-treat-asthma in adults to improve outcomes for people with severe asthma and to act as clinical gatekeepers to ensure appropriate access to high-cost technologies [Citation40].
Finally, it can be speculated that the impact of anti-IL-5 treatment would be even more pronounced in patients with absent asthma control exclusively due to eosinophilic inflammation, similarly to the observed improvement in outcomes since the first negative studies in patients, which were not selected based on blood or sputum eosinophil counts [Citation41]. Indeed, the subgroup analyses suggested a more pronounced effect in patients with higher eosinophil counts, a higher exacerbation rate at enrolment, and among patients on GINA step 5 treatment. Based on our results, prescribing anti-IL-5 treatment with either mepolizumab or reslizumab to patients correctly diagnosed with severe eosinophilic asthma according to published clinical guidelines [Citation3] seems cost-effective as it will significantly reduce exacerbation rate and hospitalizations and may alleviate OCS-related systemic side effects.
Conclusions
Mepolizumab and reslizumab provide significant and clinically relevant improvement in exacerbation rate and OCS reduction, whereas improvement in FEV1, asthma control, and asthma-related quality of life is below MCIDs. Indirect inter-study comparisons revealed no differences between the anti-IL-5 drugs in efficacy or safety measures, whilst differences in OCS reduction could not be investigated due to the lack of reslizumab studies with this outcome. Neither of the available studies incorporated novel standards of systematic assessment of difficult-to-treat asthma prior to onset of treatment. To optimise use of healthcare resources, an increasing focus on systematic assessment to differentiate difficult-to-treat asthma from severe asthma before commencing biological agents is developing.
Supplemental Material
Download MS Word (99.7 KB)Acknowledgments
We thank Jan Odgaard-Jensen, Diana Odrobináková, Heidi Møller Johnsen, Anne Kirkebjerg Due, Anne Holm Hansen, Dan Pedersen, Pernille Printzlau, Charlotte Ulrik, Lone Agertoft, and Karin Dahl Assing for their support with this work.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here
Additional information
Funding
References
- From the Global Strategy for Asthma Management and Prevention, Global Initiative for Asthma (GINA) 2017. Available from: http://www.ginasthma.org/
- Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43:1–15.
- Porsbjerg C, Ulrik C, Skjold T, et al. Nordic consensus statement on the systematic assessment and management of possible severe asthma in adults. Eur Clin Respir J. 2018;5:1440868.
- von Bülow A, Kriegbaum M, Backer V, et al. The prevalence of severe asthma and low asthma control among Danish adults. J Allergy Clin Immunol Pract. 2014;2:759–767.
- Luskin AT, Chipps BE, Rasouliyan L, et al. Impact of asthma exacerbations and asthma triggers on asthma-related quality of life in patients with severe or difficult-to-treat asthma. J Allergy Clin Immunol Pract. 2014;2:544.e2-552.e2.
- Braman SS. The global burden of asthma. Chest. 2006;130:4S12S.
- Varsano S, Segev D, Shitrit D. Severe and non-severe asthma in the community: a large electronic database analysis. Respir Med. 2017;123:131–139.
- Anderson GP. Endotyping asthma: new insights into key pathogenic mechanisms in a complex, heterogeneous disease. The Lancet. 2008;372:1107–1119.
- Amini-Vaughan ZJ, Martinez-Moczygemba M, Huston DP. Therapeutic strategies for harnessing human eosinophils in allergic inflammation, hypereosinophilic disorders, and cancer. Curr Allergy Asthma Rep. 2012;12:402–412.
- Nixon J, Newbold P, Mustelin T, et al. Monoclonal antibody therapy for the treatment of asthma and chronic obstructive pulmonary disease with eosinophilic inflammation. Pharmacol Ther. 2017;169:57–77.
- Brown P, Brunnhuber K, Chalkidou K, et al. How to formulate research recommendations. BMJ. 2006;333:804–806.
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535–b2535.
- Danish Medicines Council. Process and methods guide – how the Danish medicines council assesses several medicines within the same therapeutic area [Internet]. 2017 [cited 2018 Mar 16]; Available from: http://www.medicinraadet.dk/media/5204/process-and-methods-guide-how-the-danish-medicines-council-assesses-several-medicines-within-the-same-therapeutic-area.pdf
- Danish Medicines Council. Protokol for medicinrådets kliniske vurdering af biologiske lægemidler til svær astma [Internet]. 2017 [cited 2018 Mar 16]; Available from: http://www.medicinraadet.dk/media/4976/protokol-for-klinisk-vurdering-af-biologiske-laegemidler-til-svaer-astma_-vers10.pdf
- Danish Medicines Council. Medicinrådets behandlingsvejledning for biologiske lægemidler til svær astma [Internet]. 2018 [cited 2018 Mar 16]; Available from: http://www.medicinraadet.dk/media/7407/behandlingsvejledning-svaer-astma-10.pdf
- European Medicines Agency. Summary of products characteristics – nucala [Internet]. 2015 [cited 2018 Mar 16]; Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/003860/WC500198037.pdf
- Danish Society of Respiratory Medicine. Severe Asthma [Internet]. Dan. Lungemedicinsk Selsk.1970 [cited 2018 Mar 19]; Available from: https://www.lungemedicin.dk/
- Reddel HK, Taylor DR, Bateman ED, et al. An official American thoracic society/european respiratory society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med. 2009;180:59–99.
- Higgins JPT, Altman DG, Gotzsche PC, et al. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928–d5928.
- Bucher HC, Guyatt GH, Griffith LE, et al. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol. 1997;50:683–691.
- Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–926.
- Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371:1198–1207.
- Chupp GL, Bradford ES, Albers FC, et al. Efficacy of mepolizumab add-on therapy on health-related quality of life and markers of asthma control in severe eosinophilic asthma (MUSCA): a randomised, double-blind, placebo-controlled, parallel-group, multicentre, phase 3b trial. Lancet Respir Med. 2017;5:390–400.
- Pavord ID, Korn S, Howarth P, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet Lond Engl. 2012;380:651–659.
- Bel EH, Wenzel SE, Thompson PJ, et al., SIRIUS Investigators. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014;371:1189–1197.
- Bjermer L, Lemiere C, Maspero J, et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophil levels: a randomized phase 3 study. Chest. 2016;150:789–798.
- Castro M, Mathur S, Hargreave F, et al. Reslizumab for poorly controlled, eosinophilic asthma: a randomized, placebo-controlled study. Am J Respir Crit Care Med. 2011;184:1125–1132.
- Corren J, Weinstein S, Janka L, et al. Phase 3 study of reslizumab in patients with poorly controlled asthma: effects across a broad range of eosinophil counts. Chest. 2016;150:799–810.
- Castro M, Zangrilli J, Wechsler ME, et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respir Med. 2015;3:355–366.
- Ortega HG, Yancey SW, Mayer B, et al. Severe eosinophilic asthma treated with mepolizumab stratified by baseline eosinophil thresholds: a secondary analysis of the DREAM and MENSA studies. Lancet Respir Med. 2016;4:549–556.
- Ortega H, Chupp G, Bardin P, et al. The role of mepolizumab in atopic and nonatopic severe asthma with persistent eosinophilia. Eur Respir J. 2014;44:239–241.
- Magnan A, Bourdin A, Prazma CM, et al. Treatment response with mepolizumab in severe eosinophilic asthma patients with previous omalizumab treatment. Allergy. 2016;71:1335–1344.
- Brusselle G, Germinaro M, Weiss S, et al. Reslizumab in patients with inadequately controlled late-onset asthma and elevated blood eosinophils. Pulm Pharmacol Ther. 2017;43:39–45.
- Brusselle G, Canvin J, Weiss S, et al. Stratification of eosinophilic asthma patients treated with reslizumab and GINA step 4 or 5 therapy. ERJOpen Res. 2017 Aug 17;3(3). pii: 00004–2017. doi: 10.1183/23120541.00004-2017. eCollection 2017 Jul.
- Farne HA, Wilson A, Powell C, et al. Anti-IL5 therapies for asthma. Cochrane Database Syst Rev. 2017;9:CD010834.
- Rice JB, White AG, Scarpati LM, et al. Long-term systemic corticosteroid exposure: a systematic literature review. Clin Ther. 2017;39:2216–2229.
- Gibson PG, McDonald VM. Management of severe asthma: targeting the airways, comorbidities and risk factors. Intern Med J. 2017;47:623–631.
- von Bülow A, Backer V, Bodtger U, et al. The level of diagnostic assessment in severe asthma: a nationwide real-life study. Respir Med. 2017;124:21–29.
- Gibeon D, Heaney LG, Brightling CE, et al. British thoracic society difficult asthma network. Dedicated severe asthma services improve health-care use and quality of life. Chest. 2015;148:870–876.
- National Health Services England. Specialised respiratory services (adult) – severe asthma [Internet]. UK: National Health Service; 2017 cited 2017 Jan 1 Available from: https://www.england.nhs.uk/publication/specialised-respiratory-services-adult-severe-asthma/
- Flood-Page P, Swenson C, Faiferman I, et al. A study to evaluate safety and efficacy of mepolizumab in patients with moderate persistent asthma. Am J Respir Crit Care Med. 2007;176:1062–1071.