ABSTRACT
Introduction: Long-term non-invasive ventilation (LTNIV) for the stable hypercapnic chronic obstructive pulmonary disease (COPD)-patients have been a subject of much debate in the last two decades. The aim of this study was to compile the current knowledge on LTNIV in order to evaluate the effects on mortality and hypercapnia.
Methods: Literature search in Pubmed, Ovid, and Embase for RCTs in Humans from January 2000 through January 2019 in written English.
Results: Six studies with a total of 861 patients were included. LTNIV in stable hypercapnic COPD patients significantly reduced PaCO2 but only one study found significant reduction in mortality.
Conclusion: Our meta-analyses demonstrate that LTNIV significantly reduced PaCO2 in stable patients with chronic hypercapnic respiratory failure compared to standard care alone, and subgroup analyses on studies with a predefined plan for ventilation, showed a considerable trend towards significant reduction in mortality.
The take home messages on LTNIV in stable hypercapnic COPD are:
It is essential that the patients have stable chronic hypercapnia.
The degree of stability can best be assessed after a minimum of 2 weeks following an acute hypercapnic respiratory failure (AHRF).
It is important to ventilate the patient with the goal to reduce PaCO2 by at least 20% or below 6.5 kPa.
Introduction
Chronic obstructive pulmonary disease (COPD) is an increasingly important cause of morbidity and mortality worldwide and leads to a significant economic and social burden [Citation1]. Patients with advanced COPD often develop chronic hypercapnic respiratory failure resulting in fatigue, severe dyspnoea, increased mortality and impaired quality of life [Citation2].
Traditionally, non-invasive ventilation (NIV) has only been used in the field of pulmonary medicine to treat acute hypercapnic respiratory failure (AHRF) in patients with COPD. Several randomized controlled trials (RCTs) have documented that NIV is effective and well indicated as part of the treatment of AHRF in COPD. The risk of intubation, mortality and the hospitalization time are all reduced [Citation3].
The road towards long-term NIV (LTNIV) for stable hypercapnic COPD patients has been long and bumpy [Citation4], but two RCTs have recently shown that treatment with LTNIV at home for stable hypercapnic COPD patients reduces mortality (NNT:5) [Citation5], reduces the number of exacerbations and delays the time until next hospital admission (NNT:6) [Citation6].
The Danish Society of Pulmonary Medicine has composed a National Guideline on how to treat stable hypercapnic COPD with LTNIV. The aim of this study was to compile the current knowledge of LTNIV in order to evaluate the effects on hypercapnia and mortality.
Methods
Literature search
Literature search was performed in Pubmed, Ovid, and Embase for RCTs in humans from January 2000 through January 2019 in written English.
The following keywords were used: (COPD OR chronic obstructive pulmonary disease) AND (NPPV OR non-invasive positive pressure ventilation OR NIV OR non-invasive ventilation OR NIPPV OR home mechanical ventilation OR HMV)
RCTs in adults (18 + years old) that examined LTNIV in patients with stable hypercapnic COPD vs. standard care ± long-term oxygen treatment (LTOT) were included.
Outcomes of interest were mortality and PaCO2.
RCTs on acute COPD, other pulmonary diseases than COPD (e.g. asthma), other causes of hypercapnia (e.g. obesity hypoventilation and neuromuscular diseases) were excluded. Furthermore, cross-over trials, trials with sham-NIV and trials with a duration of LTNIV less than 4 hours/day were excluded.
Statistical analysis
Meta-analysis was conducted using metan in STATA (Version 14.2, StataCorp, College Station, TX). For binary data, relative risk (RR) estimates with 95% confidence interval (CI) were calculated, and standardized mean differences (SMD) with 95% CI were computed for continuous data. A random effects model was applied in case of large heterogeneity between the studies. Heterogeneity between studies was assessed by X2 and I2 statistics. A p-value <0.1 was considered significant heterogeneity for the X2 test. I2 values of 25%, 50%, and 75% correspond to low, moderate, and high heterogeneity [Citation7].
Results
Study inclusion
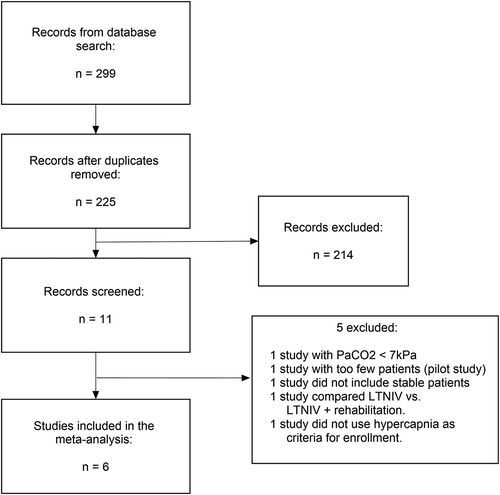
illustrates the study selection.
Included studies are listed in .
Table 1. Main characteristics of included studies.
The database search resulted in 299 studies. Among these 74 duplicates and 214 irrelevant studies were excluded. The 11 remaining studies were reduced by one study because it turned out to be a pilot study, one study because the enrolled patients were not hypercapnic, one study because the result of the arterial blood gas test was not used as a criterion for enrolment, one study because LTNIV was compared with rehabilitation instead of standard care and one study because it enrolled patients directly after discharge meaning they were not stable. No studies were solely excluded due to low compliance (<4 h hours per day).
Subsequently, six studies with a total of 861 patients were included in the meta-analysis. [Citation5,Citation6,Citation8,Citation9,Citation10,Citation11].
Carbon dioxide
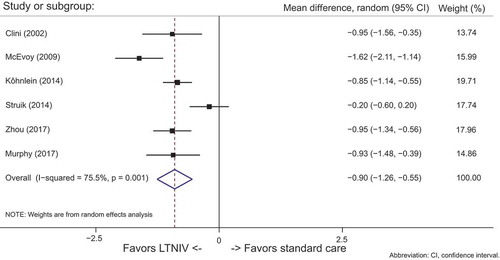
All studies in the meta-analyses contained data for PaCO2. See .
LTNIV compared to standard care significantly reduced PaCO2 in all but one of the studies. The overall reduction in PaCO2 with LTNIV was highly significant (95% CI: −1.03 to −0.69)
The high degree of heterogeneity across the studies was observed (I2 = 76%)
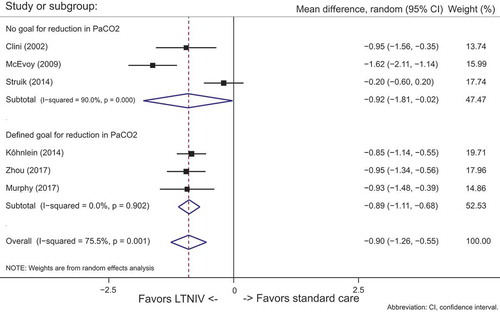
A subgroup analyses of studies with a predefined goal for reducing PaCO2 was conducted.
shows data from the subgroup analysis: Studies [Citation5,Citation6,Citation9] (n = 426) with predefined schemes for ventilation vs. studies Citation[8,Citation9,Citation10], (n = 435) with no predefined plan for ventilation. When ventilation was aimed at a specific reduction in PaCO2, the results were highly significant (95% CI: −0.90 (−1.26 to −0.55, p = 0.001).
The degree of heterogeneity in the group with a predefined goal for PaCO2 was low (I2 = 0%), compared to the group without a designated plan for ventilation (I2 = 90%).
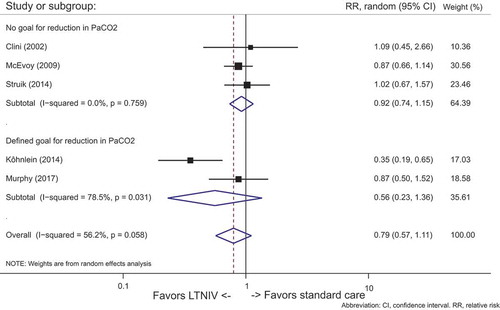
Mortality
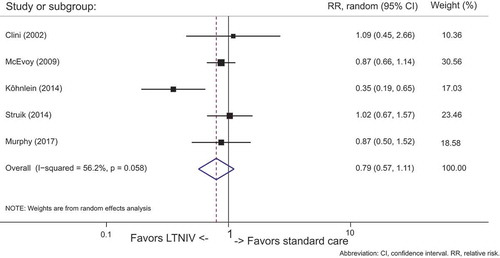
Five studies [Citation5,Citation6,Citation8,Citation9,Citation10] (n = 512) demonstrated mortality data. Data were pooled and summarized in .
In patients treated with LTNIV, only the study from Köhnlein et al. [Citation5] demonstrated significant reduction in mortality. Looking at all the studies, there was no overall significant effect on mortality (95% CI: 0.68 to 1.01, p = 0.058). Moderate heterogeneity was found (I2 = 56%).
Subgroup analyses on studies [Citation5,Citation6] (n = 311) with a predefined scheme for ventilation did not manage to show significant reduction in mortality (95% CI: 0.23 to 1.36, p = 0.031), but there was a considerable trend favoring LTNIV over standard care, compared to the studies Citation[8,Citation9,Citation10] (n = 435) with no predefined scheme for ventilation (95% CI: 0.74 to 1.5, p = 0.759). The degree of heterogeneity in the group with a predefined goal for PaCO2 was higher (I2 = 78.5%) than in the group with no predefined plan for PaCO2 (I2 = 0%). See .
Discussion
This meta-analyses of six studies with a total of 861 patients all suffering from stable hypercapnic COPD compared the efficacy of LTNIV added to standard care ± LTOT versus standard care alone ±LTOT.
Adding LTNIV to standard care resulted in a significantly reduction in PaCO2 and a trend towards reduction in mortality.
Within the last two decades, the treatment of COPD patients with NIV at home has been a hot topic and has taught us a lot on how to find the right phenotype of COPD and how to properly ventilate the patients.
The early trials on stable COPD and LTNIV did not take PaCO2 into consideration, and many of the ventilated patients were not chronic hypercapnic [Citation11].
In 2014 Struik et al. [Citation10] conducted a study with 201 hypercapnic COPD patients. Patients admitted with AHRF were enrolled almost directly after termination of ventilatory support. Stable/prolonged hypercapnia was defined as normalization of pH, but with elevated PaCO2 >48 hours after termination of ventilatory support. Unfortunately, no difference in readmission or survival was observed between the groups. One reason might be that the study population was composed of a large group of acute and not chronic hypercapnic patients, that spontaneously normalized their PaCO2. Consequently, the results were diluted by patients that were not in need of ventilatory support. In the control group, 26% of the patients had normalized their PaCO2 after 3 months. Hence, a lesson to be learned is that the definition of chronic hypercapnia should be carefully considered.
The study from Köhnlein et al. [Citation5] was published the same year but had a slightly different approach. As in the other studies stable hypercapnic patients were recruited, but a predefined ventilation scheme was included in the study. The aim was a reduction in PaCO2 defined by either a reduction of 20% or PaCO2 below 6.5 kPa, which was unique for this study. High-pressure ventilation was combined with high back up rates to achieve the predefined goals. Their patients included were slightly more hypercapnic with PaCO2 ≥7kPa.
This study was the first to demonstrate a significant reduction in mortality compared with conventional treatment. These results might partially be attributable to their predefined ventilation scheme and use of PaCO2 as a predictor for sufficient ventilation.
In 2017, Murphy et al. [Citation6] published a study including 116 semi-stable COPD patients. The patients were screened at least 2 weeks after an AHRF and were enrolled, if they still had PaCO2 >7 kPa. At the time of screening, as much as 50% of the patients had already normalized their carbon dioxide.
The study showed that adding NIV to conventional treatment made a significant improvement in admission-free survival. Murphy et al. [Citation6] used the same criteria for sufficient ventilation as Köhnlein et al. [Citation5].
The short-term study conducted by Zhou et al. [Citation9] showed significant reduction in PaCO2 when adding LTNIV to standard care in stable hypercapnic COPD patients. They did not use a predefined scheme for ventilation like Murphy et al. [Citation6] and Köhnlein et al. [Citation5]. Inspiratory positive airway pressure (IPAP) was titrated to the maximally tolerated level; however, they aimed for a pressure support of more than 10 cm H2O, ensuring some degree of minimum ventilation. LTNIV reduced PaCO2 by 17.7% from 7.7 kPa (57.78 ± 2.88 mmHg) and therefore achieved the same outcome in PaCO2 as Murphy and Köhnlein aimed for in their studies. The average IPAP was only 17.8 cm H2O. Studies [Citation12,Citation13] have previously indicated the need for high-intensity LTNIV for COPD. Nevertheless, Zhou et al. demonstrate significant reduction in PaCO2 underlining that sufficient ventilation is not a certain level of IPAP, but the right level of IPAP.
In 2013, a meta-analysis from Struik et al. [Citation4] found a significant reduction in PaCO2 over 3 months in patients with a compliance >5 hours per night, compared to compliance <5 hours per night. The Italian Multicentre study from Clini et al. [Citation8] defined compliance to LTNIV as ≥5 hours per night, and they excluded 13% (5 out of 39) due to low compliance or voluntary withdrawal. Their median daily use of LTNIV for the compliant patients was 9 ± 2 h. Zhou et al. [Citation9] had a mean time of LTNIV usage of 5.6 ± 1.4 h per day. 84% (48 out of 57) exceeded the prescribed usage time of >5 hours per day and only 8,7% had a usage time below 4 h per night. Both Köhnlein and Murphy et al. [Citation5,Citation6] had a prescribed minimum usage time of 6 h per day. 65% of the patients in the study from Köhnlein exceeded that and in the study from Murphy et al. the compliance increased from 4.7 h per night at 6 weeks to 7.6 h per night at 12 months. McEvoy et al. [Citation14] had the overall lowest compliance rate with a mean adherence to LTNIV of 4.5 ± 3.2 h per night. 60% (41 out of 72) used LTNIV for more than 4 h per night. The ideal compliance for LTNIV is indeed an area for further research. Interpatient differences must be expected, but one could argue, that if PaCO2 is falling according to the predefined plan, the compliance must be adequate, regardless of the number of hours on the ventilator.
To our knowledge, this is the first meta-analyses to date, consisting only of RCTs with LTNIV in stable hypercapnic COPD-patients.
A limitation to our study is undoubtedly the difference among the RCTs on how they define the time for stable chronic hypercapnia to be present. It ranges from 48 h in Struik et al. [Citation10] to 4 weeks in Köhnlein et al. [Citation5], but as seen in the study from Murphy et al. [Citation6]. 50% of the patients normalized their carbon dioxide within 2 weeks after an AHRF needing NIV and in the control group in Struik et al. [Citation10]. more than one fourth became normocapnic within 3 months. The definition on stable hypercapnia and when to start LTNIV needmore research. A large interpatient variability must be expected, but as the present data demonstrate, we strongly recommend a minimum of 2 weeks after an AHRF before evaluating the patient.
Conclusion
Our meta-analyses demonstrate that LTNIV significantly reduces PaCO2 in stable patients with chronic hypercapnic respiratory failure compared to standard care alone, and subgroup analyses on studies with a predefined plan for ventilation, show a considerable trend towards significant reduction in mortality.
The take home messages on LTNIV in stable hypercapnic COPD are:
It is essential that the patients have stable chronic hypercapnia.
The degree of stability can best be assessed after a minimum of 2 weeks following an AHRF.
It is important to ventilate the patient with the goal to reduce PaCO2 by at least 20% or below 6.5 kPa.
Acknowledgments
We would like to thank Torgny Wilcke, Tine Peick Sonne, and Carl Nielsen for their help on the Danish National Guideline on LTNIV for COPD.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Notes on contributors
Eline K. Gantzhorn
Eline Kirstine Gantzhorn is a MD. She is the main author of the Danish national guideline on long term non invasive ventilation for COPD. She primarily works in the field of sleep apnea, chronic obstructive pulmonary disease and chronic respiratory insufficiency.
Thomas Skovhus Prior
Thomas Skovhus Prior is a MD, PhD fellow, working in the field of interstitial lung diseases and chronic obstructive lung disease. He has a special focus on health-related quality of life, comorbidities, survival and ventilation. So far, he has published 4 articles in the these areas.
Ole Hilberg
Ole Hilberg is a MD, DMSc and professor of respiratory medicine at Southern Danish University. Has been chairing the Danish respiratory society for four years. He has more than 125 peer-reviewed publications in different aspects of respiratory medicine. His main focus has been in interstitial lung disease, lung infections and COPD.
References
- Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am J Respir Crit Care Med. 2007; 195(5). DOI:10.1164/rccm.201701-0218PP.
- Heinemann F, Schmidbauer K, Budweiser S, et al. Health-related quality of life and long-term prognosis in chronic hypercapnic respiratory failure: a prospective survival analysis. Respir Res. 2007;8(1):1–7.
- Ram FS, Picot J, Lightowler J, et al. Non-invasive positive pressure ventilation for treatment of respiratory failure due to exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2004;(4):2003–2005. DOI:10.1002/14651858.cd004104.pub3
- Struik FM, Lacasse Y, Goldstein RS, et al. Nocturnal noninvasive positive pressure ventilation in stable COPD: a systematic review and individual patient data meta-analysis. Respir Med. 2014;108(2):329–337.
- Köhnlein T, Windisch W, Köhler D, et al. Non-invasive positive pressure ventilation for the treatment of severe stable chronic obstructive pulmonary disease: a prospective, multicentre, randomised, controlled clinical trial. Lancet Respir Med. 2014;2(9):698–705.
- Murphy PB, Rehal S, Arbane G, et al. Effect of home noninvasive ventilation with oxygen therapy vs oxygen therapy alone on hospital readmission or death after an acute COPD exacerbation: a randomized clinical trial. J Am Med Assoc. 2017;317(21):2177–2186.
- Higgins JPT. Measuring inconsistency in meta-analyses. Bmj. 2003;327(7414):557–560.
- Clini E, Sturani C, Rossi A, et al. The Italian multicentre study on noninvasive ventilation in chronic obstructive pulmonary disease patients. Eur Respir J. 2002;20(3):529–538.
- Zhou L, Li X, Guan L, et al. Home noninvasive positive pressure ventilation with built-in software in stable hypercapnic COPD: a short-term prospective, multicenter, randomized, controlled trial. Int J COPD. 2017;12:1279–1286.
- Struik FM, Sprooten RTM, Kerstjens HAM, et al. Nocturnal non-invasive ventilation in COPD patients with prolonged hypercapnia after ventilatory support for acute respiratory failure: a randomised, controlled, parallel-group study. Thorax. 2014;69(9):826–834.
- Casanova C, Celli B, Tost L, et al. Long-term controlled trial of nocturnal nasal positive pressure ventilation in patients with severe COPD. Chest. 2000;118(6):1582–1590.
- Dreher M, Storre JH, Schmoor C, et al. High-intensity versus low-intensity non-invasive ventilation in patients with stable hypercapnic COPD: a randomised crossover trial. Thorax. 2010;65(4):303–308.
- Windisch W, Haenel M, STorre JH, et al. High-intensity non-invasive positive pressure ventilation for stable hypercapnic COPD. Int J Med Sci. 2009;6(2):72–76.
- McEvoy RD, Pierce RJ, Hillman D, et al. Nocturnal non-invasive nasal ventilation in stable hypercapnic COPD: a randomised controlled trial. Thorax. 2009;64(7):561–566.