ABSTRACT
Background and Objective: Despite improved asthma and chronic obstructive pulmonary disease (COPD) management, treatment remains inadequate in many patients. Understanding the impact of current treatment in settings outside of controlled trials would add important clinical decision-making information. This study evaluated costs and outcomes associated with budesonide+formoterol (BF) Spiromax® initiation among real-world Swedish patients with asthma and/or COPD.
Methods:In this retrospective observational analysis of Swedish patients with asthma and/or COPD, data were collected from the National Patient Register, National Dispensed Drug Register, and Cause of Death Register 1 year before and after initiating BF Spiromax (index date). Outcomes included exacerbation occurrence, treatment patterns, inpatient care, and healthcare costs.
Results: The study included 576 patients (asthma: 51.6%; COPD: 32.8%; and asthma and COPD: 15.6%). Following BF Spiromax initiation in asthma patients, there were significant decreases in exacerbations (41.1% to 30.0%; P < 0.001), mean comorbidity-related inpatient visits (0.5 to 0.2; P < 0.001), and inpatient days (1.9 to 0.6; P = 0.006), and a trend toward fewer asthma-related inpatient visits (mean, 0.2 to 0.1; P = 0.056) and asthma-related inpatient days (mean, 0.7 to 0.3; P = 0.060). Increased inpatient utilization was observed in patients with COPD or both diagnoses. All-cause and asthma-/COPD-related medication costs decreased in all groups.
Conclusions: After switching to BF Spiromax, asthma patients had fewer exacerbations and hospital visits versus the prior year and COPD patients showed an increase in all-cause and COPD-related healthcare resource utilization. All-cause and asthma-/COPD-related medication costs decreased in all groups after switching to BF Spiromax.
Introduction
Asthma is a chronic inflammatory disease affecting the airways and characterized by variable airway obstruction. With age-dependent variations, the proportion of patients diagnosed with asthma in Sweden is 7%−10% [Citation1,Citation2]. Patients tend to be undertreated in the real-world setting and those with adequate treatment often have inadequate asthma control [Citation3].
Chronic obstructive pulmonary disease (COPD) is characterized by persistent respiratory symptoms and airflow limitation due to airway and/or alveolar abnormalities usually caused by significant exposure to noxious particles or gases [Citation4]. The prevalence of COPD in patients older than 45 years of age is 8%−14% [Citation5]. Fifty percent of elderly smokers developed COPD in one cross-sectional cohort study in Sweden [Citation5] and, even among nonsmokers, the prevalence of COPD in Sweden is 3%−8% [Citation6]. COPD is a major cause of chronic morbidity and mortality worldwide and often coincides with conditions such as cardiovascular disease, diabetes, and musculoskeletal impairment [Citation7]. Approximately 3000 people per year die from COPD-related complications in Sweden [Citation7,Citation8] and, according to the Swedish PATHOS study, COPD patients have a life expectancy 8 years shorter than the general population [Citation9].
Real-world studies have shown that incorrect inhaler use is associated with poor symptom control and worse outcomes in patients with asthma or COPD [Citation10–Citation12]. A new fixed-dose combination of the inhaled corticosteroid (ICS) budesonide and the long-acting β2-agonist (LABA) formoterol (referred to here as budesonide+formoterol [BF] Spiromax®) was granted marketing authorization by the European Medicines Agency in April 2014 for the treatment of asthma and COPD and launched in Sweden in September 2014. BF Spiromax was designed to reduce common inhaler preparation errors and enhance usability. Studies have shown ease of use for patients and healthcare professionals compared with other dry powders [Citation13,Citation14]. Clinical data demonstrated the safety and efficacy [Citation13] of BF Spiromax, but effectiveness data in a real-world setting are limited. The objective of this study was to obtain real-world evidence among Swedish patients on the treatment, management, outcomes, and cost of asthma and COPD associated with use of BF Spiromax.
Materials and methods
Study design
This study was a retrospective analysis using the administrative records of patients with asthma and/or COPD who were receiving secondary care for any kind of disease and who initiated BF Spiromax in Sweden between September and December 2014. The total study period was between 1 January 1997 and 31 December 2015. The study focused on patients with asthma and/or COPD who initiated BF Spiromax and who had been treated with the following ICS+LABA inhalers: Accuhaler/DiskusTM, Turbuhaler®, other ICS+LABA devices (>1 prescription), or had ≤1 prescription of an ICS+LABA before the initiation of BF Spiromax). Each patient’s observational period comprised the 1-year ‘baseline’ period prior to the first prescription of BF Spiromax (index date) and the 1-year ‘follow-up’ period after the index date. Only patients who were alive at the end of the follow-up period were included in the analysis.
Registers used
Data were obtained from national health registers. The National Patient Registry (NPR) was used to collect inpatient and outpatient data from all the hospitals in Sweden. The NPR dates back to 1964; from 1987, there is information on all completed in-patient admissions in publicly operated hospitals; from 2001, collection of outpatient care data began. The NPR covers 99% of all somatic and psychiatric discharges in Sweden and is updated annually [Citation15]. For this study, the NPR was used to identify all eligible patients with COPD and/or asthma and to generate comorbidity and events information traced to individual patients. In order to retain a full year of follow-up, data from the Hospital Discharge Register and Hospital Outpatient Register up to the end of 2015 were extracted.
All medications prescribed and dispensed to patients are tracked in the National Dispensed Drug Register (NDR), which covers all pharmacy transactions from 1 July 2005. In this study, the NDR was used to identify the population of BF Spiromax users and the data on COPD- and asthma-specific treatments and concomitant medications. Data extracted from the register included: patient identifier, age, sex, residency, date of dispensed prescription, number of packages dispensed, defined daily dose of package, total cost, product name for all drugs.
The Cause of Death Registry (CDR) includes data on all deaths covering all residents in the country, whether or not the individual was a Swedish citizen, and irrespective of whether or not the deaths occurred in any of the Nordic countries. In this study, the CDR was used to collect data on patient identifier, date of death and primary cause of death.
Variables were derived from the available data sources and were collected and linked using the National Board of Health and Welfare through personal identification numbers.
Patients included
Three patient populations were identified for the study using diagnostic codes – those with asthma alone, those with asthma and COPD diagnosis codes, and those with COPD alone. All patients had at least one claim for an asthma- and/or COPD-related visit to secondary care (with a primary or secondary asthma/COPD diagnosis).
Patients in the asthma only group were aged ≥18 years at first dispensation of BF Spiromax (index date), and had an asthma diagnosis (J45/J46) and no record of COPD/emphysema diagnosis (J43/J44) during the total study period (1997 − 2014). Patients in the asthma/COPD group were aged ≥40 years at index date and had both an asthma and a COPD/emphysema diagnosis during the total study period. Patients in the COPD only group were aged ≥40 years at index date with a COPD/emphysema diagnosis (J43/J44) during the study period and no record of asthma diagnosis during the study period.
Assessment of outcomes
Study outcomes included exacerbations (defined as an inpatient visit or dispensary of oral steroids or antibiotics [COPD] or inpatient visit or dispensary of oral steroids [asthma]), utilization of asthma/COPD treatments, hospital visits, and medication costs one year before initiation of BF Spiromax and one year after this treatment was started. The index date was included in the baseline period for all variables except treatments and medication costs. The outcomes were measured for the total population and in the subgroup of patients who continued using BF Spiromax for the entirety of the follow-up period. The occurrence of exacerbations was also assessed in the subgroup who had ≤1 ICS+LABA prescription during the 1-year baseline period (Accuhaler/Diskus; Turbuhaler; other). All-cause and asthma/COPD-related inpatient and outpatient utilization was assessed. Hospital visits are reported as the mean number of inpatient days as well as the mean number of inpatient visits. All-cause and asthma/COPD-related medication costs are reported in euros.
Statistical analyses
Statistical analyses were carried out using the SAS 9.3 software package, with the level of significance set at 0.05. Continuous variables were expressed as means, standard deviations, minimums, medians, maximums, and quartiles, while categorical variables were expressed as the number of cases per category and in terms of relative frequencies. McNemar’s test was used for the occurrence of treatments and exacerbations and paired t-tests were used for hospital visits and treatment costs. Appropriate diagnostics were conducted to ensure requirements were met for the parametric tests that were used.
Role of the funding source
This study was funded by Teva Pharmaceuticals, as was the medical writing support. The study design and the analysis and interpretation of the data were performed as a collaboration of the funder and the authors. The sponsor provided a full review of the article. The authors were fully responsible for all content and editorial decisions and were involved at all stages of manuscript development; they have all approved the final version.
Results
Patients
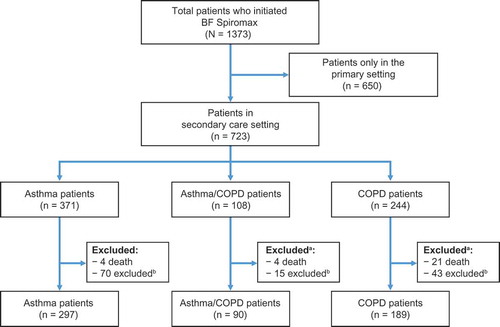
From the NDR search 1,373 patients were prescribed BF Spiromax between September and December 2014 and, of those, 723 patients were identified through a secondary care setting and were eligible for the analysis (). Therefore, approximately 47% of patients identified in the total population receiving BF Spiromax were managed entirely in primary care, never received a diagnosis in secondary care, and were not included in the analysis. Of the patients eligible for the study, 297 patients with asthma alone, 90 patients with asthma and COPD, and 189 patients with COPD alone were included in the study ().
Figure 1. Patient flow diagram. aPatients who were excluded due to adherence criteria, and who died prior to the end of the follow-up period are listed in both of the exclusion categories; bPatients who were excluded, were excluded due to lack of adherence. To be included, all patients had to be on BF Spiromax for the entire follow-up, with a minimum of two prescriptions. BF: budesonide+formoterol; COPD: chronic obstructive pulmonary disease.
Baseline characteristics indicate that each of the study populations had a slightly higher percentage of women (59.6% in asthma, 58.9% in asthma/COPD, and 58.2% in COPD; ). When age was broken down by decades, the most common age category was 60 − 69 years for asthma and asthma/COPD (18.9% and 35.6%, respectively) and 70 − 79 years for COPD (36.5%). The most common baseline ICS+LABA regimen was Turbuhaler (39.4%, 45.6%, and 49.7% in the asthma, asthma/COPD, and COPD groups, respectively).
Table 1. Patient characteristics among an eligible patient population.
Exacerbations
Of the 297 patients in the asthma group, 17% switched to another ICS+LABA. A significant decrease in the percentage of patients who had an exacerbation following a BF Spiromax prescription, from 41.1% to 30.0%, was observed in the total population (P < 0.001) as well as among all patients who did not change to another ICS+LABA (40.1% to 27.1%; P = < 0.001) and among patients who had ≤1 ICS+LABA prescription during the baseline period (37.7% to 23.9%; P = 0.003; )). No significant between-group differences were observed in the analysis stratified by baseline ICS+LABA treatment (data not shown).
Figure 2. Percentage of patients with an exacerbation before and after initiating BF Spiromax for the asthma patient group (a), the asthma/COPD patient group (b), and the COPD patient group (c). BF: budesonide+formoterol; COPD: chronic obstructive pulmonary disease; ICS: inhaled corticosteroid; LABA: long-acting β2-agonist.
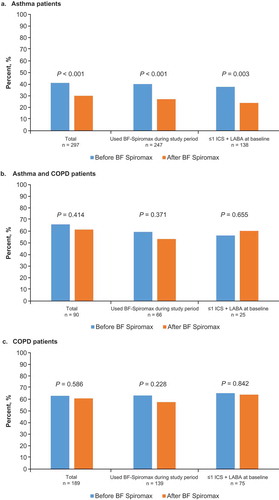
Of the 90 patients in the asthma/COPD group, 27% changed to using another ICS+LABA. No significant difference in exacerbations was observed after initiating BF Spiromax in this patient group ()).
Of the 189 patients in the COPD alone group, 26% changed to use another ICS+LABA. No significant difference in exacerbations was observed after initiating BF Spiromax in the COPD alone group ()).
Treatment patterns
Among patients in the asthma group, SABA use significantly decreased from 59.9% to 51.2% (P = 0.003) for the total study population (); among those patients who did not change to another ICS+LABA, it decreased from 58.3% to 47.4% (P < 0.001) (data not shown). No difference was observed in short-acting muscarinic antagonist (SAMA) or LAMA use among patients in this patient group. In addition, no significant difference in SABA, SAMA, or LAMA use was observed among patients in either the asthma/COPD group or the COPD group.
Table 2. Asthma/COPD treatments before and after initiation to BF Spiromax in the total population.
Hospitalizations
Inpatient and outpatient utilization is shown in .
Figure 3. Inpatient and specialized outpatient care before and after initiating BF Spiromax in patients with asthma (a), asthma/COPD (b), or COPD (c). BF: budesonide+formoterol; COPD: chronic obstructive pulmonary disease.
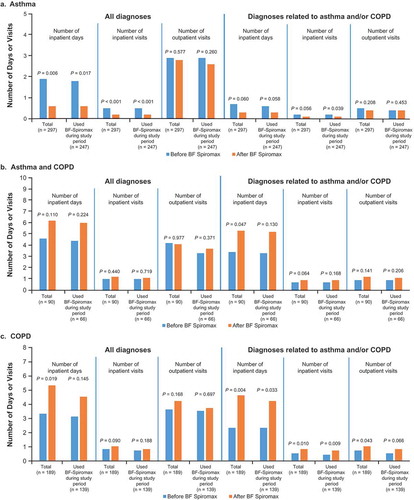
In the asthma group, mean all-cause inpatient visits decreased significantly from 0.5 to 0.2 per patient (P < 0.001) and mean all-cause inpatient days decreased from 1.9 days to 0.6 (P = 0.006), with a similar pattern observed in patients who did not change to another ICS+LABA ()). Similar differences were observed for asthma-related inpatient visits; but the differences did not reach significance except for mean asthma-related inpatient visits (0.2 to 0.1 per patient; P = 0.039). No significant differences in all-cause or asthma-related outpatient visits were observed.
In the asthma/COPD group, no significant difference was seen for all-cause inpatient utilization (inpatient visits or days). However, there was a significant increase in mean asthma/COPD-related inpatient days from 3.4 to 5.3 (P = 0.047) ()). A similar pattern of differences was observed in patients who did not change to another ICS+LABA, however none of the differences reached significance. No significant differences in all-cause or asthma/COPD-related outpatient visits were observed.
In the COPD group, there was a significant increase in all-cause inpatient days (3.3 to 5.3 days; P = 0.019), with a similar pattern (albeit the differences were not significant) observed for patients who did not change to another ICS + LABA ()). No significant differences in all-cause inpatient visits were observed. There was a significant increase in COPD-related inpatient visits from 0.5 to 0.8 (P = 0.0097), and a significant increase in COPD-related inpatient days from 2.3 to 4.6 (P = 0.004). Similar significant differences were seen in patients who did not change to another ICS+LABA. A significant increase from 0.7 to 1.0 in COPD-related outpatient visits in the total population (P = 0.043) was observed.
Medication and secondary care costs
Medication costs and secondary care costs are shown in .In the asthma group, there were significant decreases in all-cause medication costs from €676.9 to €587.5 (P < 0.001) and for asthma-related medication costs from €663.9 to €572.2 (P < 0.001). Patients who did not change to another ICS+LABA showed a similar pattern ()). All-cause secondary care costs significantly decreased from €2951.0 to €1638.1 (P < 0.001) in the total population and from €2918.8 to €1459.4 (P < 0.001) in patients who did not change to another ICS+LABA. A similar pattern was seen for asthma-related secondary care costs, however the decreases observed were not statistically significant.
Figure 4. Cost of medications (units of euros) and secondary care before and after initiating BF Spiromax in patients with asthma (a), Asthma and COPD (b) or COPD (c). BF, budesonide+formoterol; COPD, chronic obstructive pulmonary disease.
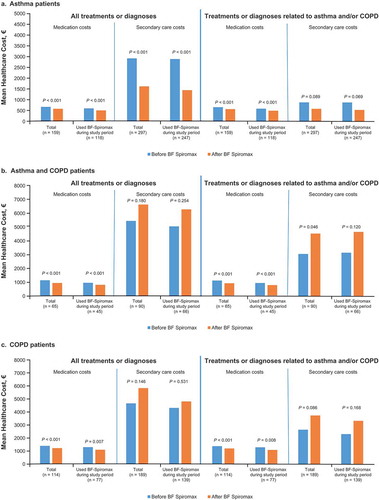
In the asthma/COPD group, all-cause medication costs decreased from €1175.3 to €967.6 (P < 0.001) in all patients and from €981.6 to €823.2 (P < 0.001) in those who did not change to another ICS+LABA. Asthma/COPD-related medication costs decreased from €1153.5 to €945.8 (P < 0.001) in all patients and from €960.0 to €993.6 (P < 0.001) in patients who did not change to another ICS+LABA ()). All-cause secondary care costs increased but the differences did not reach significance. Mean asthma/COPD-related secondary care costs in the total population significantly increased from €3043 to €4519 (P = 0.046).
In the COPD group, all-cause medication costs decreased from €1291.3 to €1125.6 (P < 0.001) in all patients and from €1195.9 to €1005.4 (P = 0.007) in those who did not change to another ICS+LABA. COPD-related medication costs decreased from €1267.0 to €1098.7 (P < 0.001) in all patients and from €1185.6 to €993.6 (P = 0.008) in those who did not change to another ICS+LABA ()). All-cause and COPD-related secondary care costs increased but the differences did not reach significance.
Discussion
In patients with asthma, BF Spiromax was associated with a statistically significant reduction in exacerbations and inpatient resource utilization from the baseline year. In addition to the reduction in exacerbations and resource utilization, there was a significant reduction in both treatment costs and secondary care costs following the initiation of BF Spiromax.
Patients with COPD, with or without asthma, did not experience statistically significant changes from baseline in exacerbations and showed statistically significant increases in healthcare resource utilization. In the current analysis, adjustments were not made for comorbidities and other baseline characteristics. Additionally, patients in the asthma/COPD may actually have had a prior misdiagnosis of asthma prior to their COPD diagnosis. The high rate of tiotropium use in the asthma/COPD cohort supports this possibility. Our analysis was not able to detect or account for misdiagnosis.
Exacerbations and costs were decreased in patients with asthma alone. There was no significant change in the numbers of patients with exacerbations in the COPD group; however, statistically significant increases in hospitalizations and secondary care costs were seen. One potential explanation is that an increase in inpatient utilization for the asthma/COPD and COPD groups may reflect the natural history of COPD because worsening disease is associated with high comorbidity. A prolonged length of hospital stay for patients with COPD has been associated with increased comorbidities and the increased healthcare costs of patients with COPD have been attributed to comorbid conditions [Citation7]. However, we are not able to definitively explain the differences between disease cohorts in this analysis. Results in all the disease cohorts should be interpreted with caution because of the absence of a control group of patients who stayed on the same ICS+LABA during the baseline and follow-up year. Furthermore, the analysis did not control for baseline factors that might influence exacerbation risk, utilization, or cost and there was limited statistical power in the subgroup analyses. Patients changing to another treatment during an inpatient or outpatient secondary care visit may have more severe or unstable disease compared with the overall population of patients with asthma, COPD, or asthma/COPD treated with an ICS+LABA.
Another important limitation of the study was that the analyses were restricted to patients identified from secondary care records because of the limited availably of primary care data in Swedish health registries. In Sweden, the vast majority of asthma and COPD patients are treated in the primary care setting and, so, the necessity of using secondary care records to identify patients and outcomes may have introduced bias because comorbidities may be overrepresented. Therefore, it is possible that patients with milder disease may have been underrepresented. However, many patients in the study were seeking secondary care for reasons not associated with asthma or COPD and, presumably, these patients’ asthma and/or COPD were managed in the primary care setting. Nonetheless, it is unknown whether these results can be generalized to patients managed entirely in primary care, and may not be directly comparable with country-specific database studies in which primary care records are included.
The major strength of the study was use of the well-managed, large, Swedish National Health Registries database [Citation15] In addition, the time horizon of 1-year baseline and 1-year follow-up minimized the impact of potential seasonal differences in disease activity. In this analysis, real-world treatment outcomes were assessed, including the occurrence of exacerbations, treatment patterns, inpatient care, and treatment costs. Real-world effectiveness may differ from clinical trial results because registration trials are performed in a controlled setting with restrictive inclusion/exclusion criteria and a high degree of patient support and monitoring. Data obtained in clinical settings outside of controlled trials adds important information to support clinical decision-making.
In conclusion, asthma patients who switched to BF Spiromax experienced fewer exacerbations and hospital visits compared with the year prior to switching. BF Spiromax appeared effective in a population derived from real-world clinical practice, in agreement with the results of clinical trials. Medication costs for all disease cohorts were driven by asthma/COPD medication and were lower after switching to BF Spiromax. Patients with COPD showed an increase in all-cause and COPD-related healthcare resource utilization, which was not associated with a statistically significant increase in secondary care costs. Results of this descriptive analysis should provide direction for further research on the effects of inhalers with better ease of use on healthcare resource utilization and costs.
Declarations of Interest
Christer Janson has received consultancy or speaker fees from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Meda, Novartis, and Teva Pharmaceuticals; Hicham Benhaddi is an employee of Teva Pharmaceuticals and owns stock in the company; Michael Törnblom and Milica Uhde are employees of IQVIA, contracted to plan and perform this study; Gunnar Johansson has served as a consultant for Teva Pharmaceuticals and AstraZeneca.
Acknowledgments
Medical writing support was provided by Melissa Kirk, PhD, of Ashfield Healthcare Communications, part of UDG Healthcare plc, and was funded by Teva Pharmaceuticals.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Notes on contributors
Christer Janson
Christer Janson is a pulmonologist and Professor in Respiratory Medicine at the Department of Medical Sciences for Respiratory; Allergy, and Sleep Research, Uppsala University, Uppsala, Sweden. He is a member of the Swedish and European Respiratory Societies. His research has focused on asthma and COPD.
Hicham Benhaddi
Hicham Benhaddi is Director of Global Health Economics & Outcomes Research at Teva Pharmaceuticals, Wilrijk, Belgium, where he has worked for nearly 5 years. Hicham has 15 years experience in HEOR and Market Access. He was educated at the Université de Bourgogne and the Université Paris Dauphine from which he received two Master’s degrees in Health Economics.
Michael Törnblom
Michael Törnblom is a biostatistician at IQVIA based in Stockholm, Sweden. He is currently conducting research within real-world evidence in various therapeutic areas.
Milica Uhde
Milica Uhde has over 13 years of research experience, including her Ph.D. studies at Karolinska Institutet in Stockholm, where her thesis focused on the metabolic effects of thyroid hormones. Dr. Uhde joined IQVIA in 2015, and has since managed numerous observational and post authorization safety studies in the fields of oncology/hematology and respiratory, using data from EMRs and Scandinavian national health registries. She holds an M.Sc in molecular pathophysiology from Södertörns University in Stockholm.
Gunnar Johansson
Gunnar Johansson is a specialist in family and internal medicine. He is also Professor Emeritus in the Department of Public Health and Caring Services, Family Medicine and Preventative Medicine at Uppsala University, Uppsala, Sweden. His research has focused on asthma and COPD.
References
- Bjerg A, Ekerljung L, Middelveld R, et al. Increased prevalence of symptoms of rhinitis but not of asthma between 1990 and 2008 in Swedish adults: comparisons of the ECRHS and GA2LEN surveys. PLoS One. 2011;6(2):e16082.
- Ekerljung L, Bjerg A, Bossios A, et al. Five-fold increase in use of inhaled corticosteroids over 18 years in the general adult population in West Sweden. Respir Med. 2014;108(5):685–10.
- Global Initiative for Asthma (GINA). GINA report, global strategy for asthma management and prevention. 2017. [cited 2019 Aug 7]. Available from: https://ginasthma.org
- Global Initiative for Chronic Obstructive Lung Disease. Pocket guide to COPD diagnosis, management, and prevention: a guide for health care professionals. Edition. 2017. [cited 2019 Aug 7]. Available from: https://goldcopd.org
- Lundbäck B, Lindberg A, Lindström M, et al. Obstructive lung disease in northern Sweden studies. Not 15 but 50% of smokers develop COPD? — Report from obstructive lung disease in northern Sweden studies. Respir Med. 2003;97(2):115–122.
- Hagstad S, Backman H, Bjerg A, et al. Prevalence and risk factors of COPD among never-smokers in two areas of Sweden – Occupational exposure to gas, dust or fumes is an important risk factor. Respir Med. 2015;109(11):1439–1445.
- Negewo NA, Gibson PG, McDonald VMCOPD. and its comorbidities: impact, measurement and mechanisms. Respirology. 2015;20(8):1160–1171.
- Swedish National Board of Health and Welfare Statistical database. [cited 2019 Aug 7]. Available from: https://www.socialstyrelsen.se/en/statistics-and-data/statistics/statistical-database
- Ställberg B, Janson C, Johansson G, et al. Management, morbidity and mortality of COPD during an 11-year period: an observational retrospective epidemiological register study in Sweden (PATHOS). Prim Care Respir J. 2014;23(1):38–45.
- Lavorini F, Magnan A, Dubus JC, et al. Effect of incorrect use of dry powder inhalers on management of patients with asthma and COPD. Respir Med. 2008;102(4):593–604.
- Al-Jahdali H, Ahmed A, Al-Harbi A, et al. Improper inhaler technique is associated with poor asthma control and frequent emergency department visits. Allergy Asthma Clin Immunol. 2013;9(1):8.
- Price DB, Román-Rodríguez M, McQueen RB, et al. Inhaler errors in the CRITIKAL study: type, frequency, and association with asthma outcomes. J Allergy Clin Immunol Pract. 2017;5(4):1071–1081.r9.
- Virchow JC, Rodriguez-Roisin R, Papi A, et al. A randomized, double-blinded, double-dummy efficacy and safety study of budesonide-formoterol Spiromax compared to budesonide-formoterol Turbuhaler in adults and adolescents with persistent asthma. BMC Pulm Med. 2016;16:42.
- Bosnic-Anticevich S, Callan C, Chrystyn H, et al. Inhaler technique mastery and maintenance in healthcare professionals trained on different devices. J Asthma. 2018;55(1):79–88.
- Ludvigsson JF, Andersson E, Ekbom A, et al. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11:450.
