ABSTRACT
Background: Maximum exercise workload (WMAX) is today assessed as the first part of Cardiopulmonary Exercise testing. The WMAX test exposes patients with COPD, often having cardiovascular comorbidity, to risks. Our research project was initiated with the final aim to eliminate the WMAX test and replace this test with a predicted value of WMAX, based on a prediction algorithm of WMAX derived from multicentre studies.
Methods: Baseline data (WMAX, demography, lung function parameters) from 850 COPD patients from four multicentre studies were collected and standardized. A prediction algorithm was prepared using Random Forest modelling. Predicted values of WMAX were used in a new WMAX test, which used a linear increase in order to reach the predicted WMAX within 8 min. The new WMAX test was compared with the standard stepwise WMAX test in a pilot study including 15 patients with mild/moderate COPD.
Results: The best prediction algorithm of WMAX included age, sex, height, weight, and six lung function parameters. FEV1 and DLCO were the most important predictors. The new WMAX test had a better correlation (R2 = 0.84) between predicted and measured WMAX than the standard WMAX test (R2 = 0.66), with slopes of 0.50 and 0.46, respectively. The results from the new WMAX test and the standard WMAX test correlated well.
Conclusion: A prediction algorithm based on data from four large multicentre studies was used in a new WMAX test. The prediction algorithm provided reliable values of predicted WMAX. In comparison with the standard WMAX test, the new WMAX test provided similar overall results.
Introduction
Spirometry is mandatory for establishing a diagnosis of chronic obstructive pulmonary disease (COPD) but is not sufficient for a general clinical assessment and evaluation of potential treatment effects. Functional performance assessed at walk or bicycle exercise tests together with measurements of symptoms and health-related Quality of Life (QoL) provide valuable additional information about present disease status, as well as prediction of future risk of exacerbations and disease prognosis [Citation1–Citation7]. The standard bicycle exercise protocol for cardiopulmonary exercise testing (CPET) measures the endurance time of cycling during a standard endurance test, at a constant rate of 75% of the maximum exercise workload (WMAX) [Citation7–Citation11]. WMAX is obtained in a preceding incremental WMAX test, in which the patient is subjected to a stepwise increase in workload until the point of physical exhaustion or another symptom-limited reason for stopping is reached.
CPET is technically demanding and requires expensive equipment [Citation11]. A considerable number of participants are needed to detect potential therapeutic effect differences due to high inter- and intra-test variability of the standard endurance test [Citation8,Citation10,Citation12,Citation13]. In addition, CPET is associated with a certain increase in cardiac risks, particularly at the initial WMAX test [Citation7,Citation8].
A way to address the above downsides would be to correctly predict WMAX, which optimally could avoid the WMAX test by using 75% of the predicted value for the endurance test. Several papers have identified predictors for WMAX or maximal oxygen consumption (VO2MAX) in COPD patients using the standard WMAX or VO2MAX test. Forced expiratory volume in 1 sec (FEV1) has been identified as an important predictor [Citation14–Citation19]. Other predictors identified are static lung volumes (e.g. inspiratory capacity; IC) [Citation14,Citation15,Citation18], small airway dysfunction, e.g. reduced mid-expiratory flow (FEF25-75) [Citation17,Citation20,Citation21], impaired gas exchange measured by e.g. diffusing capacity for carbon monoxide (DLCO) [Citation14,Citation15,Citation18,Citation20,Citation22], maximal voluntary ventilation (MVV) [Citation18,Citation19,Citation21], Medical Research Council (MRC) scale [Citation16,Citation17], sub-maximal exercise [Citation23,Citation24] and demographic data such as sex, age, body-mass index (BMI) or fat-free mass [Citation14–Citation17,Citation20,Citation21,Citation25]. However, another article concluded that predicted VO2MAX from baseline characteristics cannot be used for patients with stable COPD [Citation26].
Previous papers claiming predictability of WMAX (VO2MAX) had different reasons for trying to replace the incremental test by a predictive (WMAX or VO2MAX) value, e.g. to determine an individual patient’s future risks of, e.g., exacerbations, to avoid complication in a cardiopulmonary risk population, to reduce costs caused by expensive equipment and to achieve a reasonably accurate endurance value for a rehabilitation program. Only one study [Citation27] has specifically addressed the possibility to predict the individual incremental step increase to keep the test to 8–10 min (see also [Citation16,Citation28]). Most previous prediction papers have used stepwise and not linear increase. A number of older studies concluded that a linear ramp test was no better than a stepwise incremental test (for an overview, see [Citation8]), but these papers could be challenged.
We, therefore, embarked on a research project aiming to improve CPET by
elimination of the incremental WMAX test in order to avoid the cardiovascular risks associated with the test and, in addition, save clinical study resources. Instead, a carefully predicted value of WMAX is to be used to identify the starting point for the 75% endurance test.
decreasing the usually high intra- and inter-variability of the 75% endurance test and thereby offer comparative COPD study designs needing less patients.
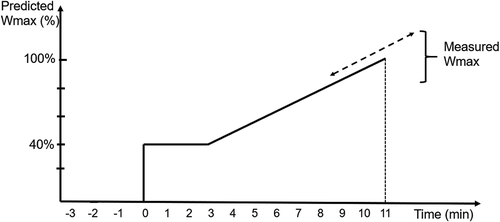
As an initial step, we designed a new model for the incremental WMAX test involving three combined ideas to be tested: 1) a prediction algorithm, developed from four large pharmaceutical industry-sponsored clinical studies, in order to identify a predicted value of WMAX to be reached within a certain exercise time (3 + 8 min; see ); 2) a linear instead of stepwise increase of workload to introduce continuous WMAX values instead of categorical values of 10 W per step. 3) measurements of future predictors (e.g. low-intensity O2/CO2 kinetic evaluation, impulse oscillometry and activity scores).
The proposed new WMAX test was compared with the standard incremental WMAX test [Citation8] in a single-centre pilot study. This report includes two major parts – the development of the prediction algorithm and the results from the pilot study.
The primary objective of the pilot study was to verify the prediction algorithm using the new WMAX test. The secondary objective was to show that the new WMAX test provided better results compared to the standard WMAX test, for use in future studies within this research project. This new WMAX test will be utilized until the prediction model provides values of WMAX that are reliable enough to be used as the starting point for the 75% endurance test.
Materials and methods
Studies used for developing a prediction algorithm of WMAX
Patients
Baseline data (demography, lung function parameters and WMAX from incremental exercise tests) were provided from two pharmaceutical companies (AstraZeneca and Boehringer Ingelheim) via standard data-sharing agreements. Patients with COPD participated in four multicentre, randomised clinical studies examining the effects of tiotropium (Study A,B,D) [Citation12,Citation13,Citation29] or budesonide/formoterol (Study C) [Citation30] on exercise performance (). The inclusion criteria were similar in Study A-C, while Study D included patients with milder COPD. Patients were clinically stable patients with COPD, aged >40 years, with a smoking history of >10 pack-years, a pre-bronchodilator FEV1 ≤ 65% (Study A and B) of predicted normal, ≤50% (Study C) or 50–80% (Study D). Studies were approved by medical ethical committees and all patients gave written informed consent before undertaking any study procedures.
Table 1. Patient baseline characteristics per study for study A-D and the pilot study
Study design
In Study, A-D eligibility criteria were assessed during an initial screening visit. At this or a following pre-randomization visit, patients performed pulmonary function tests followed by a standard incremental WMAX test. Study A-C were conducted using bicycle exercise testing while Study D used treadmill exercise testing.
The data were combined into a single dataset following transformations in order to account for differences in units, data formats and naming of the variables. Note that all variables were not available from all studies.
Outcome measures
For the post hoc analysis of Study A-D, the outcome was a prediction algorithm for the best possible prediction of WMAX.
Statistical analyses
Prediction algorithms were formulated using Random Forest modelling [Citation31] for each of Study A-D separately. The Random Forest model was used to automatically handle potential non-linear associations among the variables (predictors). A list of the ranking order among the variables was obtained, sorted in descending order with the best predicting variable first. Random Forest modelling was performed using R (v 3.5.1 for Mac) software. Univariate regression was performed in Microsoft Excel for Office 365. The Random Forest algorithms are available for external use upon request.
Pilot study to compare new and standard WMAX test
Patients
In the pilot study, patients with COPD who had no exacerbations of COPD within the last 6 weeks, a post-bronchodilator FEV1 of ≥40 to ≤80% of predicted normal and no cardiovascular co-morbidity preventing exercise testing were included. The Regional Ethical Review Board approved the study. Written informed consent was obtained from all patients prior to any study procedures. Patients were out-patients who had previously visited our clinic and registered for volunteering in other research studies.
Study design
The study was non-blinded and single-centre. At visit 1, demography data, modified medical research council (MMRC) dyspnea scale scores and clinical COPD questionnaire (CCQ) scores were collected. The COPD patients performed lung function tests (spirometry, body plethysmography, CO-diffusion test and impulse oscillometry) after inhalation of 400 µg salbutamol. The predicted value of WMAX for each patient was calculated on a pre-programmed computer holding the Random Forest algorithm.
Patients then performed the standard or the new WMAX test in a random cross-over fashion at the following visits. During the standard test, after a few minutes of sitting on the bicycle in order to stabilize the oxygen kinetics measurement equipment, the patient had an approximately one-minute warm-up period of loadless pedalling, followed by an incremental increase in workload with 10 W per minute until the patient reached his/her WMAX at the point of exhaustion. During the new WMAX test, the patients started cycling at a load of 40% of the predicted WMAX for 3 min, followed by a linear increase in load, calculated to reach the predicted WMAX after an additional 8 min (). In both WMAX tests, the same safety procedure were applied, as recommended for the standard WMAX test [Citation7,Citation8]. Borg scale results were collected at every 2 min during both tests [Citation32].
Outcome measures
The primary outcome measure was the ability of the prediction algorithm to successfully predict WMAX as shown by the coefficient of determination (R2) between predicted and measured WMAX at the new WMAX test. Secondary outcome variables were descriptive comparisons between the new and the standard WMAX test for averages of measured WMAX and work performed, reasons for stopping exercise and Borg scale results.
Statistical analyses
We designed the study to include 15–20 patients in order to obtain enough data to test the prediction model and to compare the new test with the standard test, but no formal power calculation was performed. The comparisons between predicted and measured WMAX, and the new and the standard WMAX test were performed using univariate regression, descriptive statistics and Bland–Altman graphs [Citation33]. Microsoft Excel for Office 365 was used for univariate regression analyses and Bland–Altman graphs. Demographics and patient data were expressed as mean ± standard deviation.
Results
Studies used for developing a prediction algorithm of WMAX
In total, 850 patients with COPD were included in the dataset for Study A-D, and their baseline data are summarized in . Using Random Forest modelling, all lung function and demographic variables for each of Study A-D were assessed for their ability to predict WMAX. Results are presented in . The best percentage of variance explained was obtained for Study A, followed by Study B, C and D. The six best lung function variables in Study A were FEV1, DLCO, forced expiratory flow at 50% (FEF50), forced vital capacity (FVC), alveolar volume (VA) and FEF25-75. Random Forest modelling for Study B-D () showed that FEV1 was the highest ranked variable in all three studies. In patients with the most severe COPD disease (Study C), predictors after FEV1 were FVC, vital capacity (VC), IC and residual volume (RV). In Study D (the study with subjects with milder COPD disease), FEV1 was followed by IC, FVC and DLCO. In Study B, FEV1, FVC and VC dominated as predictors. Neither Study B nor Study C included measurements of DLCO. Absolute values showed better results than % of predicted values. Univariate regression analyses of measured WMAX versus individual values for each variable are presented in .
Table 2. Rank order for prediction ability for different variables from Random Forest modelling in study A-D
Table 3. Results from univariate regression analyses of individual values from each parameter versus measured Wmax in study A-D and the pilot study
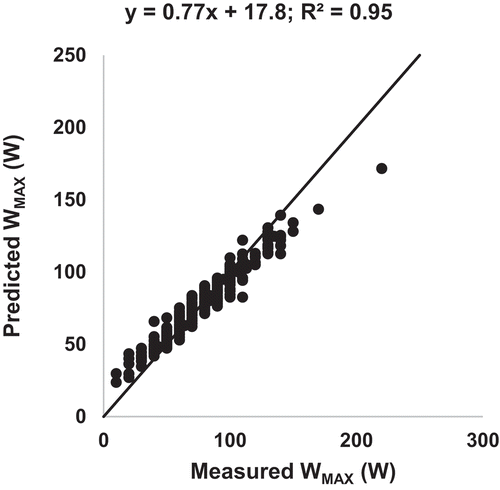
shows that DLCO, together with FEV1, was the best predictor in Study A. It was decided to base the prediction algorithm on the results from this study only, since the other study with measurements of DLCO (Study D) was a study using treadmill walking in patients with mild COPD. The lung function variables listed above from Study A were selected for the prediction algorithm, together with age, sex, height and weight. The results from the prediction algorithm are presented in the , where predicted WMAX has been plotted versus measured WMAX for Study A. An R2-value of 0.95 and a slope of 0.77 was observed.
Pilot study to compare new and standard WMAX test
Patient population
A total of 19 patients were enrolled between July and October 2015. Fifteen of these were included in the study. Three were excluded due to FEV1 ≥ 80% predicted and one due to significant cardiovascular co-morbidity. Fifteen patients with COPD were included in the pilot study. They had a mean age of 71 years and mean FEV1 was 60% predicted normal. Further patient baseline characteristics are presented in .
Ability of the prediction algorithm to predict WMAX
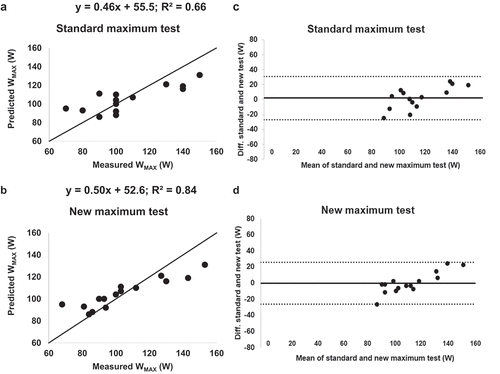
shows the correlation of predicted WMAX versus measured WMAX for the two tests () plus the corresponding Bland–Altman plots (). Predicted values of WMAX correlated well with measured WMAX for the new test (R2 = 0.84). For the standard test, a smaller determination coefficient was observed (R2 = 0.66), even though the prediction algorithm was based on the standard WMAX test performed in Study A. The slope for predicted WMAX versus measured WMAX was 0.50 for the new test and 0.46 for the standard test.
Comparison of the standard and new WMAX test
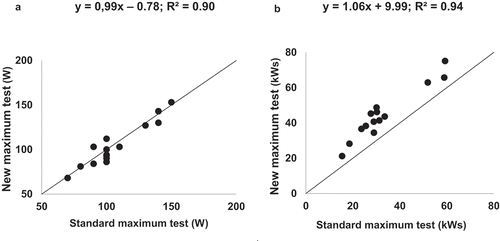
The prediction algorithm from Study A was used for the new WMAX test. Similar average results were obtained for WMAX, work capacity, time of exercise, Borg scale results and reasons for stopping exercise at the two tests (). For WMAX reached (, part A) and work performed in kWs (, part B), R2-values of 0.90 (WMAX) and 0.95 (work capacity) were observed when the standard test and the new test were compared. A higher amount of work with a lesser percentage deviation was achieved during the new WMAX test (47.5 kWs ± 36% SD) than during the standard WMAX test (35.5 kWs ±45% SD) (). The average duration was 9.3 ± 2.6 min (excluding the initial 3 min at 40% of predicted WMAX) for the new test compared to 10.6 ± 2.6 min for the standard test.
Table 4. Results of the standard maximum test and new maximum test performed in the pilot study
Significance of DLCO
DLCO was the variable with the highest R2-value from univariate regression (0.78; ) in the pilot study, which demonstrates its high ability for prediction of WMAX in a small single-centre study. In Study A (a multi-centre study), univariate regression gives a mean R2-value of 0.37 for DLCO, but with a large inter-centre variability (range from 0.01 to 0.83 for the individual centres; data not shown).
Discussion
In our search for a prediction algorithm with the ability to accurately predict WMAX to a level where standard incremental WMAX test can be eliminated, we investigated baseline lung function and demographic data together with results from a standard incremental WMAX test from four large pharmaceutical industry-sponsored clinical studies. Since DLCO was found to have outstanding abilities for prediction of WMAX, a prediction algorithm was built using Random Forest modelling of data from only one of these four studies, a multicentre study with 261 patients. FEV1 was another an excellent predictor, as was also observed in the three remaining studies, but these studies either lacked measurements of DLCO or used treadmill rather than bicycle exercise testing. The obtained prediction algorithm was utilized to set the low-intensity start and the linear increase in the new linear WMAX test in a pilot study. This study compared the new WMAX test with the standard incremental WMAX test. The prediction algorithm was successful in providing reliable values of predicted WMAX, and the measured value of WMAX from the new test correlated better with the predicted value than did the corresponding value from the standard test. The R2-value (our primary variable) was 0,84 for predicted vs measured WMAX for the new test. It should, however, be noted that the new WMAX test will possess the same cardiovascular risks as the standard WMAX test.
In comparison with the standard WMAX test, the new WMAX test provided similar overall results for work capacity, time of exercise, Borg scale results and reasons for stopping exercise. The slope for predicted WMAX versus measured WMAX was 0.50 (new test) and 0.46 (standard test), respectively, indicating that, for both tests, WMAX in the high range obtained from high performers are underestimated by the prediction algorithm and that WMAX in the low range (low performers) are overestimated by the prediction algorithm. The conformity was best for patients with mid-range WMAX values and hence the prediction algorithm needs further improvement. An improved prediction algorithm would improve the precision of the new WMAX test, so that the endurance test will be performed at an optimal level. If the patient starts the endurance test at too low or too high values as derived from the WMAX test, too long or too short endurance times may be observed leading to an increased intra-test variability.
The two tests correlated well with measured WMAX, but the new linear test gave continuous data (not 10W stepwise data) along the regression line and achieved the predicted value of WMAX in 9.3 ± 2.4 min (programmed value 8 min), which meant that the increase was adapted to the respective individual. This resulted in a higher amount of performed work with less deviation. The standard test and the new test showed similar mean WMAX. but the amount of work performed in kWs was constantly higher for the new method for both low and high performers. This is partly explained by the initial 3 min of bicycling at 40% of WMAX, but this accounts only for some 30–40% of the difference. The rest of the difference may be explained by the individualized increase in work overtime. In the standard test the load started at 10 W (but can be 30–40 W based on the experience of the investigator). The incremental steps were 10 W (but steps from 5 to 20 W have been utilized), and higher increases than 10 W have been pointed out to be beneficial [Citation8]. In the new test, the initial low load and the following linear increase to WMAX are personalized. The bicycle is programmed to reach the predicted WMAX in 8 min; i.e. a predicted high performer faces a much steeper increase than a predicted low performer. Also, the precision of WMAX is lower in the standard test, since work performed is calculated based on 10W-increments. This is exemplified in where five patients have a measured WMAX of 100 W in the standard test.
A large number of prediction formulas for WMAX or VO2MAX in COPD patients have been presented [Citation14–Citation25,Citation27,Citation28]. Most of these have been derived from single-centre studies with low numbers of patients, and the generalizability of these formulas to broader COPD populations and to multicentre studies can be questioned. We took another approach by using data from large multicentre studies sponsored by pharmaceutical companies. The resulting prediction algorithm might be more accurate and generalizable as compared to most previous prediction equations of WMAX due to the bigger and broader COPD populations included in the analyses. However, the multicentre data includes many centres with different qualities in performing a correct WMAX test. For DLCO, problems with standardisation across centres and large coefficients of variation have been observed [Citation34]. A good and generalizable prediction algorithm could be helpful to achieve the best possible measured WMAX in order to have the 75% level in the endurance test as accurate as possible.
One of our major learnings is that the single and multicentre approach identified very similar predictors. Our prediction algorithm identified FEV1 (flow), DLCO (gas-exchange), FVC and VA (volume), FEF50 (small airways) and demographic data, which agrees well with previous publications [Citation14–Citation25,Citation27,Citation28]. One study [Citation26] criticised the use of baseline lung function to predict VO2MAX However, this study selected six prediction formulas from three papers [Citation14,Citation15,Citation22] of which four included transplant candidates and one very severe COPD patients while the validation was done in 60 COPD-patients equally divided into GOLD 2, 3 and 4. Other papers have not challenged the possibility of predicting WMAX or VO2MAX. However, Fregonezi [Citation26] highlight a very important issue of all prediction models, i.e. generalisability, both related to disease severity and quality of assessment between centres.
The current study has a number of limitations. The prediction algorithm has been based on multicentre studies with high variability. Only one study was finally used as some of the key variables for prediction have not been collected in the other studies. A limitation with Random Forest is the non-intuitive relationship between the different variables making a judgement based on clinical knowledge important. In addition, Random Forest is usually applied to larger sample sizes. In the studies, the measurements of WMAX and the demographic parameters were well documented but other good predictors had a lower quality or were missing (e.g. DLCO). The prediction algorithm does not account for differences in the level of activity among the patients, and whether the patients have little or much experience from bicycling. Also, the low number of patients in the pilot study makes the results from this study sensitive to the performance by individual patients.
Areas for further improvements of the prediction algorithm include QoL-variables, variables measuring daily activities, oxygen kinetic data and values from examinations of small airway functions such as impulse oscillometry measures. The possible role of hyperinflation as predictors in more severe patients should also be explored [Citation35].
In summary, a prediction algorithm for WMAX has been developed from incremental exercise bicycle tests in previously published multicentre clinical studies. The best predictors were FEV1 and DLCO. A new linear WMAX test including the use of the prediction algorithm correlated well with the standard incremental WMAX test. The new WMAX test had a better correlation to the prediction algorithm, even though the algorithm was developed from the standard WMAX test. In addition, an improved prediction algorithm with a slope closer to line-of-identity will benefit the new WMAX test. Therefore, the new test will be utilized in future studies within our research project until the prediction algorithm has reached a level of precision that enables the WMAX test to be replaced with a predicted value of WMAX.
Acknowledgments
We thank Boehringer Ingelheim and AstraZeneca for providing patient-level data for this post-hoc analysis, Dr Ulf Nihlén, Department of Respiratory Medicine and Allergology, University Hospital, Lund, Sweden for valuable comments and the study nurses at the Department of Respiratory Medicine and Allergology, University Hospital, Lund, Sweden for their assistance during the pilot study.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Chawla H, Bulathsinghala C, Tejada JP, et al. Physical activity as a predictor of thirty-day hospital readmission after a discharge for a clinical exacerbation of chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2014;11(8):1203–10.
- Iyer AS, Wells JM, Bhatt SP, et al. Life-space mobility and clinical outcomes in COPD. Int J Chron Obstruct Pulmon Dis. 2018;13:2731–2738.
- Marin JM, Alfageme I, Almagro P, et al. Multicomponent indices to predict survival in COPD: the COCOMICS study. Eur Respir J. 2013;42(2):323–332.
- Marin JM, Carrizo SJ, Casanova C, et al. Prediction of risk of COPD exacerbations by the BODE index. Respir Med. 2009;103(3):373–378.
- Moy ML, Teylan M, Weston NA, et al. Daily step count predicts acute exacerbations in a US cohort with COPD. PLoS One. 2013;8(4):e60400.
- Rodriguez DA, Kortianou EA, Alison JA, et al. Heart rate recovery after 6-min walking test predicts acute exacerbation in COPD. Lung. 2017;195(4):463–467.
- Palange P, Ward SA, Carlsen KH, et al. ERS task force.recommendations on the use of exercise testing in clinical practice. Eur Respir J. 2007;29(1):185–209.
- American Thoracic Society. American College of Chest P. ATS/ACCP Statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2003;167(2):211–277.
- O’Donnell DE, Lam M, Webb KA. Measurement of symptoms, lung hyperinflation, and endurance during exercise in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;158(5 Pt 1):1557–1565.
- Puente-Maestu L, Palange P, Casaburi R, et al. Use of exercise testing in the evaluation of interventional efficacy: an official ERS statement. Eur Respir J. 2016;47(2):429–460.
- Singh S, Harvey-Dunstan TC. Walking for the assessment of patients with COPD. In: Palange P, Laveneziana P, Neder JA, et al., editors. Clinical exercise testing (ERS monograph). Sheffield: European Respiratory Society; 2018. p. 175–195.
- Magnussen H, Paggiaro P, Schmidt H, et al. Effect of combination treatment on lung volumes and exercise endurance time in COPD. Respir Med. 2012;106(10):1413–1420.
- Maltais F, Hamilton A, Marciniuk D, et al. Improvements in symptom-limited exercise performance over 8 h with once-daily tiotropium in patients with COPD. Chest. 2005;128(3):1168–1178.
- Cahalin L, Pappagianopoulos P, Prevost S, et al. The relationship of the 6-min walk test to maximal oxygen consumption in transplant candidates with end-stage lung disease. Chest. 1995;108(2):452–459.
- Carter R, Holiday DB, Stocks J, et al. Predicting oxygen uptake for men and women with moderate to severe chronic obstructive pulmonary disease. Arch Phys Med Rehabil. 2003;84(8):1158–1164.
- Hartman JE, Slebos DJ, Boezen HM, et al. Selecting the increment size for a maximal incremental cycle test in patients with COPD. Respirology. 2015;20(2):352–355.
- Inal-Ince D, Savci S, Coplu L, et al. Functional capacity in severe chronic obstructive pulmonary disease. Saudi Med J. 2005;26(1):84–89.
- Carter R, Peavler M, Zinkgraf S, et al. Predicting maximal exercise ventilation in patients with chronic obstructive pulmonary disease. Chest. 1987;92(2):253–259.
- LoRusso TJ, Belman MJ, Elashoff JD, et al. Prediction of maximal exercise capacity in obstructive and restrictive pulmonary disease. Chest. 1993;104(6):1748–1754.
- Behnia M, Wheatley C, Avolio A, et al. Influence of resting lung diffusion on exercise capacity in patients with COPD. BMC Pulm Med. 2017;17(1):117.
- Efremidis G, Tsiamita M, Manolis A, et al. Accuracy of pulmonary function tests in predicted exercise capacity in COPD patients. Respir Med. 2005;99(5):609–614.
- Chuang ML, Lin IF, Vintch JR. Comparison of estimated and measured maximal oxygen uptake during exercise testing in patients with chronic obstructive pulmonary disease. Intern Med J. 2004;34(8):469–474.
- Coquart JB, Eston RG, Lemaitre F, et al. Prediction of peak oxygen uptake from ratings of perceived exertion during a sub-maximal cardiopulmonary exercise test in patients with chronic obstructive pulmonary disease. Eur J Appl Physiol. 2015;115(2):365–372.
- Ekblom-Bak E, Bjorkman F, Hellenius ML, et al. A new submaximal cycle ergometer test for prediction of VO2max. Scand J Med Sci Sports. 2014;24(2):319–326.
- Cavalheri V, Hernandes NA, Camillo CA, et al. Estimation of maximal work rate based on the 6-minute walk test and fat-free mass in chronic obstructive pulmonary disease. Arch Phys Med Rehabil. 2010;91(10):1626–1628.
- Fregonezi G, Resqueti V, Vigil L, et al. Maximal oxygen uptake cannot be estimated from resting lung function and submaximal exercise in patients with chronic obstructive pulmonary disease. J Cardiopulm Rehabil Prev. 2012;32(4):219–225.
- Buchfuhrer MJ, Hansen JE, Robinson TE, et al. Optimizing the exercise protocol for cardiopulmonary assessment. J Appl Physiol Respir Environ Exerc Physiol. 1983;55(5):1558–1564.
- Debigare R, Maltais F, Mallet M, et al. Influence of work rate incremental rate on the exercise responses in patients with COPD. Med Sci Sports Exerc. 2000;32(8):1365–1368.
- O’Donnell DE, Maltais F, Porszasz J, et al. The continuum of physiological impairment during treadmill walking in patients with mild-to-moderate COPD: patient characterization phase of a randomized clinical trial. PLoS One. 2014;9(5):e96574.
- Worth H, Forster K, Eriksson G, et al. Budesonide added to formoterol contributes to improved exercise tolerance in patients with COPD. Respir Med. 2010;104(10):1450–1459.
- Couronne R, Probst P, Boulesteix AL. Random forest versus logistic regression: a large-scale benchmark experiment. BMC Bioinformatics. 2018;19(1):270.
- Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14(5):377–381.
- Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307–310.
- Hegewald MJ, Markewitz BA, Wilson EL, et al. Single-breath diffusing capacity for carbon monoxide instrument accuracy across 3 health systems. Respir Care. 2015;60(3):430–436.
- Eriksson G, Jarenback L, Peterson S, et al. A new approach to assess COPD by identifying lung function break-points. Int J Chron Obstruct Pulmon Dis. 2015;10:2193–2202.