ABSTRACT
Background: High-intensity resistance training is unexplored in untreated patients with newly diagnosed sarcoidosis.
Objectives: To evaluate the effects of high-intensity resistance training on lung function, muscle strength, fatigue, dyspnea, health-related impairments, and lung immune cells.
Methods: Eleven untreated patients with newly diagnosed sarcoidosis performed high-intensity resistance training at an intensity of 80% of 1 Repetition Maximum (RM) twice a week and daily inspiratory muscle training at regular intensity for 12 weeks. Assessment with spirometry, chest X-ray, questionnaires, and BAL (bronchoalveolar lavage) cells was performed before and in close adjacent to completed training. A final third assessment except bronchoscopy was performed at an average 5 months after the training period.
Results: The training was well tolerated and muscular strength increased significantly while fatigue, dyspnea, and health-related impairments decreased, though not significantly in all measures. Mean percentage of lung lymphocytes decreased (p = 0.006).
Conclusions: High-intensity resistance training and inspiratory muscle training at regular intensity in patients with newly diagnosed sarcoidosis led to improvements in muscular strength without adverse events and seems to be a non-invasive attractive way to improve fatigue, dyspnea, and quality of life. Analysis of lung immune cells possibly indicated a decreased inflammatory activity. These results provide a basis for larger randomized trials.
Introduction
Sarcoidosis is an inflammatory disorder which can affect any organ, but the lung is the most common target. Two clinical phenotypes are recognized: Löfgren´s syndrome (LS) and non-Löfgren´s syndrome (non-LS). LS is characterized by an acute onset with a high likelihood of spontaneous remission. Patients with non-LS often present with a more gradual onset and are more likely to develop chronic disease [Citation1]. Certain human leukocyte antigen (HLA) class II alleles are linked to a non-resolving disease [Citation2]. In the lungs, an increased cell concentration and accumulation of CD4+ T cells are seen. BAL has a valuable role in the diagnostic workup and differential diagnosis of sarcoidosis [Citation3]. Increased BAL cell concentration, an accumulation of CD4+ T cells, and a CD4/CD8 ratio exceeding 3,5 strongly support the diagnosis of sarcoidosis [Citation4]. There is no cure and despite treatment, patients often have non-specific complaints such as exercise intolerance, muscle weakness, and fatigue, which do not seem to correlate with lung function, inflammatory markers, and other clinical parameters. Not even dyspnea has been found to correlate with lung function [Citation5,Citation6]. Instead, research has found a correlation between muscle strength, fatigue, dyspnea, and quality of life [Citation7–Citation9] and that patients with sarcoidosis have lower muscle strength compared to healthy controls [Citation5,Citation8,Citation10–Citation14]. A newly published paper also reported that physical activity is significantly reduced in patients with sarcoidosis compared to healthy controls [Citation15]. In many chronic lung diseases and inflammatory disorders, physical training has been shown to improve exercise intolerance, muscle weakness, quality of life, and reduce fatigue without adverse events [Citation16] and limited data indicates that inflammatory activity is reduced [Citation17–Citation20]. Also in sarcoidosis, fatigue, muscular weakness, and dyspnea are reduced while quality of life is improved after participating in training programs [Citation7,Citation9,Citation21,Citation22]. However, data is scarce [Citation23] and no study investigated the effect on inflammatory parameters and in studies performed so far, patients with various disease durations, both treated and untreated have been included. The purpose of the current study was to explore if high-intensity resistance training is safe, improves health-related impairments, if the lung sarcoid inflammation is affected and possible effects on disease course in newly diagnosed patients.
Materials and methods
Study design
Participants were identified amongst consecutive patients with a high suspicion of sarcoidosis referred to the Department of Respiratory Medicine, Karolinska University Hospital, Stockholm, Sweden. At the first visit, treatment-naive non-smoking patients between 20 and 60 years of age without signs of other pulmonary (e.g. COPD, asthma), cardiac, chronic inflammatory, or metabolic diseases were asked if they were interested to participate if a diagnosis of sarcoidosis could be established. Patients with LS were excluded as that disease phenotype often resolves spontaneously. The study was approved by the Regional Ethical review Board (2015/763-31) in Stockholm. Upon enrollment, information on the study was given both orally and written. All participants signed an informed consent according to the declaration of Helsinki. Twelve patients were enrolled in the study and completed a general somatic evaluation including blood samples for CRP, ACE, HLA DRB1*-typing, a 12-lead electrocardiogram, questionnaires, and bronchoscopy with BAL. The bronchoscopy was done within a couple of weeks after referral. In cases where the sarcoidosis diagnosis was still unclear after bronchoscopy, the patients were referred to mediastinal lymph node puncture via esophagus. All included patients fulfilled the criteria for sarcoidosis according to the World Association of Sarcoidosis and Other Granulomatous Disorders [Citation1,Citation24]. Spirometry and ergospirometry were performed using a SensorMedics Vmax system (SensorMedics, Yorba Linda, CA, USA). A second assessment including blood samples, spirometry, questionnaires, and bronchoscopy was made within 1–2 months after the training was completed. A third assessment except bronchoscopy was made on average 5 months after finalized training.
Bronchoscopy and questionnaires
Bronchoscopy with BAL was performed as previously described [Citation25]. For individual details on BAL procedures, see Appendix A. Cells in BALF (bronchoalveolar lavage fluid) were separated from recovered fluid by centrifugation, fixed on cytospin slides and stained with Giemsa for calculation of leukocyte differential count. The percentage of CD4+ and CD8+ T cells, respectively, was measured by triple-laser, eight-color flow cytometry using a FACS Fortessa X-20 (Becton-Dickinson).
Quality of life was assessed using the Swedish version of St George´s Respiratory Questionnaire (SGRQ), containing three component items. These can be aggregated into a total score. Scores are expressed as a percentage of overall impairment where 100 represents the worst possible health status. A difference of at least 4 points in the SGRQ total score is considered a minimum clinically important difference from the patient’s perspective [Citation26]. Means for SGRQ scores in normal subjects with no history of respiratory disease are 12 for S, 9 for A, 2 for I, and 6 for T (SGRQ manual, Prof Paul Jones, Division of Cardiac and Vascular Science, St George´s, University of London, UK). Fatigue was measured with the Fatigue Severity Scale (FSS). Maximal score 63 indicates a great impact of fatigue. A total score of less than 36 suggests that the patient does not suffer from fatigue. The FSS has been found to reliably capture fatigue impact in a variety of clinical populations [Citation27]. Physical Activity Level Scale was used to estimate physical activity. This scale has been used in several studies concerning the effects of different types of intervention. Activity levels during summer and winter were recorded separately. Level 1 means hardly any activity at all while level 6 reflects hard/very hard exercise regularly and several times a week [Citation28]. The 6-point (0–5) modified Medical Research Council (mMRC) dyspnea score was used to determine dyspnea. A higher point means more dyspnea. This scale has been most widely used in COPD but has also been shown to be reliable in interstitial lung disease for the assessment of dyspnea [Citation29]. The Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines recommended using mMRC score of 2 as the symptomatic cut-off point.
Training
The high-intensity resistance training followed current guidelines [Citation30] and started on average 2.6 months after the firs bronchoscopy and was performed twice a week for 60 min during 12 weeks under supervision of an experienced physiotherapist. If a training session was missed, the period was prolonged. The training consisted of a 10-min warm-up on a stationary bicycle and 9 high-intensity resistance exercises. The training intensity was 80% of 1 RM. Exercises were performed in three sets. An increase in load was applied when the individual could perform three sets of >7 repetitions. At the start and end of the training period, 1 RM for each of the exercises was calculated. Respiratory muscle strength was assessed using an electronic pressure transducer (Micro RPM, Intramedic AB, Bålsta, Sweden). Maximum inspiratory and expiratory pressures (MIP and MEP) were measured. Patients were instructed in inspiratory muscle training using a pressure threshold-loading device (Threshold IMT). The training used regular resistance, i.e. this part of the training was not of high-intensity, and started at a load of 30% of MIP and was performed 30 × 3 twice daily at a level correlating to 12–14 on Borg Rating of Perceived Exertion [Citation31].
Data analysis
Statistical analyses were performed using IBM SPSS Statistics 24 software. Power analysis was not performed given the exploratory nature of the study. Descriptive statistics, i.e. mean and standard deviation (SD) for ratio data, was performed. Wilcoxon´s signed-rank test was applied to assess changes in muscle strength, MIP, MEP, BALF data, lung function parameters, and questionnaires. P-value significance was set at <0.05.
Results
Study subjects
Twelve patients (ten men and two women) were included in the study. One (number 12) dropped out in the middle of the training due to lack of time. Data from this patient was excluded from the analysis. Clinical characteristics of patients are shown in . Nine of the patients were positive for HLA DRB1* alleles that have been associated with chronic disease [Citation2,Citation32]. Assessment of overall physical activity with Physical Activity Level Scale resulted in a mean value of 3.4 and 3.3 during summer and winter, respectively. All patients had a normal resting electrocardiogram and ergospirometry found no signs of cardiac disease. CRP levels were around normal in all patients throughout the study period. Mean ACE level decreased from 57 E/l (reference <70) to 51 after training (p > 0.05). Adherence to high-intensity resistance training supervised by a physiotherapist and follow-up assessments was good (Appendices B–D). Protocols for inspiratory muscle training relieved that adherence was not as good as for the supervised training (not shown). No major adverse events were recorded, but some minor complaints (detailed information given in Appendix E). Patient number 11 started to smoke during the study, this was not discovered until the follow-up bronchoscopy, so it was decided to keep patient´s data in the study. Patient number 3 disclosed a progress on chest X-ray at first follow-up, therefore the patient was put on systemic treatment and did not participate in the last follow-up. At second follow-up, three more patients disclosed a progress. Progress was minor and none of the patients needed treatment. Two patients disclosed a regress at follow-ups. See Appendix E for individual details.
Table 1. Baseline characteristics
Lung immune cells and spirometry
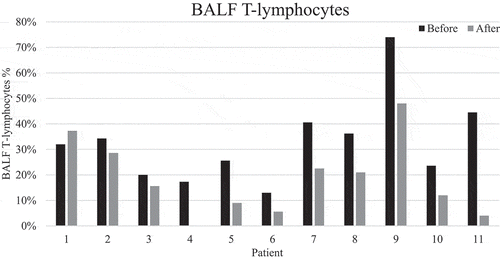
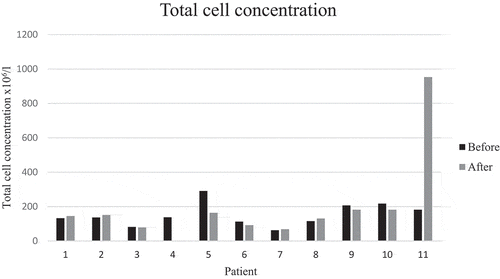
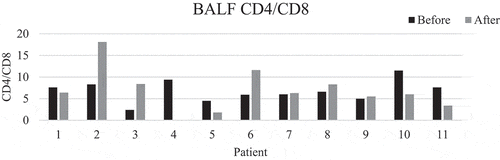
There was a significant decrease in mean percentages of T lymphocytes, from 33 to 20% (p = 0.006), corresponding to an increase in the mean percentage of macrophages. Mean CD4/CD8 ratio increased from 6.5 to 7.6 (p > 0.05). The ratio disclosed large individual differences between baseline and follow-up ( and ). There were no significant changes in neutrophils, eosinophils, basophils, and mast cells. The main results remained the same even when the patient who had started smoking was removed from the analysis. This patient differed very much from the rest in total cell concentration with a large increase at follow-up (). The change in mean total cell concentration was not significant regardless of the inclusion or exclusion of this patient. Three patients had a TLC and two patients a FEV1 less than 80% of predicted. However, mean lung function parameters (percent of predicted) were within normal limits and did not change significantly between baseline and follow-ups, see (second follow-up not shown). No correlation was seen with mMRC scores. Patient number 5 who had no respiratory symptoms at all was one of the patients with the lowest lung function values with TLC 65% and FEV1 76% of predicted at baseline.
Table 2. Lung function parameters
Figure 1. CD4/CD8 ratio before and after training. Patient number 4 did not undergo bronchoscopy after training
Questionnaires
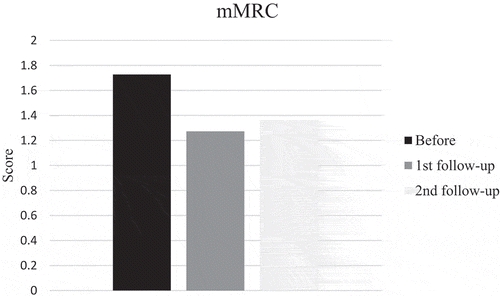
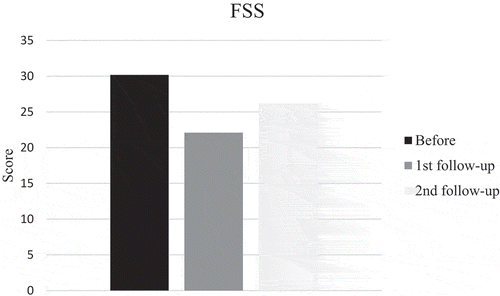
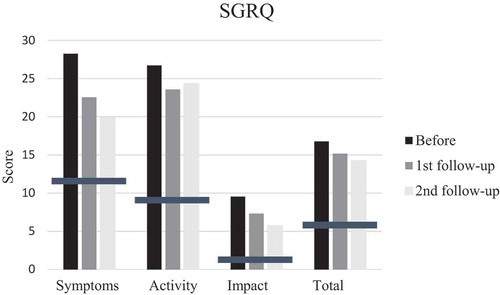
SGRQ and mMRC scores pointed in direction of improvement at first follow-up after training, but did not reach significance while FSS scores improved significantly (p = 0.035). Also the patient with disease progress improved. However, the mean score before training was 30, indicating that the patients did not suffer much from fatigue and only four patients had scores of 36 or more. Mean mMRC score before training was less than 2, thus it seems as if the patients did not suffer from much dyspnea. But, when looking at individual levels, seven patients had a score of 2 or more. Changes in scores at second follow-up were small and not significant (–).
Figure 4. Mean scores of St George´s Respiratory Questionnaire (SGRQ) items; S (symptoms), A (activity), impact (I) and T (total score) before, at first and second follow-up after training. Bars indicate means for SGRQ in normal subjects with no history of respiratory disease
Training
Results from 1 RM tests, MIP and MEP are presented in . The mean increase was significant for all exercises. Even the patient with disease progress at first follow-up disclosed an improvement in all exercises.
Table 3. Results from 1 Repetition Maximum (RM), maximal inspiratory (MIP), and expiratory (MEP) pressure tests before and after training
Discussion
This is the first study to explore the effect of a high-intensity resistance training program in patients with newly diagnosed sarcoidosis. The results indicate that training is safe, does not augment the sarcoid inflammation, leads to increased muscular strength, decreased fatigue, and may improve health-related impairments and dyspnea.
No serious adverse events were recorded, only some minor complaints. This is in line with a Cochrane review from 2014 [Citation16] focusing on training programs for interstitial lung diseases where no adverse events were identified. In fear of worsening patient´s symptoms, previous studies have used submaximal training [Citation7,Citation33]. The present study could not detect any signs of worsening symptoms. It must be stressed though that our study sample is limited with a gender imbalance, that included patients had a moderate disease activity and a relatively normal lung function, and that the observation period was rather short. However, it is notable that nine of the patients were positive for HLA-DRB1* alleles that are associated with chronic disease outcome [Citation2]. Only one out of eleven patients disclosed a progress at first follow-up. However, also this patient benefitted from the training with reduction of fatigue and health-related impairments while muscular strength improved. At present, it is unclear how lung immune cells change over time in patients with untreated sarcoidosis. Thus, when we suggest that our data points towards a decreased inflammation, our conclusion is drawn from comparison with BALF lung immune cells in healthy controls [Citation25]. One study investigated lung immune cells in BALF after recovery of sarcoidosis (>2 years after initial presentation) and disclosed a decrease in lymphocytes, CD4/CD8 ratio, and cell concentration [Citation34]. In that study, only patients with LS were investigated, whereas our study only included non-LS patients and the second bronchoscopy in our study was performed earlier in the disease course. Therefore, we are unable to draw major conclusions from BALF data. A possible positive effect of physical exercise in people with sarcoidosis is likely to be complex and depend on several pathways. Experimental studies performed on mice have shown that training induces alterations in peripheral blood lymphocyte subpopulations [Citation35] and in mice with obesity-related airway hyper-responsiveness, training induced a reduction of several inflammatory cells (including lymphocytes) as well as pro-inflammatory cytokines in BALF [Citation36]. T regulatory cells (Tregs) normally dampen the release of proinflammatory cytokines and thereby have a potential function in controlling and ending immune responses. In sarcoidosis, the exaggerated inflammatory response has, at least partly, been explained by a dampened function and/or reduced frequency in BALF and blood of Tregs. Interestingly, in a mice model of asthma, physical exercise caused an increase of activated Tregs in the lungs [Citation37]. Thus, a possible explanation for the positive effect of training could be restoration of a dysregulated Treg pool.
Despite improvements in muscular strength, lung function remained unchanged, which is in line with results from previous studies. One study investigated the effect of inspiratory muscle training in sarcoidosis and reported an improvement of dyspnea but not lung function [Citation9]. In other studies regarding sarcoidosis and training, dyspnea was not specifically recorded, however fatigue and health-related impairments improved while lung function remained the same [Citation21,Citation33]. Thus, it seems as if dyspnea is not only related to lung function and the mechanisms behind the experience of dyspnea are multifactorial where fatigue, decreased physical activity, and muscular strength are important factors. Several authors have described a negative vicious circle including fatigue, skeletal muscle weakness, and exercise intolerance, leading to physical deconditioning further exaggerating symptoms including dyspnea. Physical training seems to be a way to interfere in this vicious circle [Citation5,Citation8,Citation23].
The patients with a decreased lung function were too few to allow subgroup analysis. Also, most other interventional studies performed so far, at least to our knowledge, have included patients with normal or just below normal mean lung function [Citation7,Citation9,Citation21]. Two studies investigated the effects of a 12-week exercise program in patients with lower lung function and included patients with fibrotic disease. One of them could not detect any improvement [Citation22] while the other reported a trend towards improvement of FVC [Citation33]. Findings on patients with COPD have shown improvements in dyspnea after inspiratory muscle training but results on functional tests are conflicting [Citation38–Citation40]. Also, results on lung function after exercise programs in patients with idiopathic pulmonary fibrosis are conflicting [Citation33,Citation41]. Taken together, it cannot be ruled out that sarcoidosis patients with a decreased lung function would benefit from an exercise program with regard to lung function.
It is also important to point out that we do not have knowledge about the optimal time duration or type of training. One recent study investigated patients with interstitial lung disease (including sarcoidosis) and the results showed that patients benefited more from a 12-week exercise program compared to eighth weeks [Citation42]. Thus, we can speculate that longer training periods may be even better.
The improvements in health-related impairments, dyspnea, and fatigue were not as large as we had expected, and only reached significance for fatigue. Included patients had a newly diagnosed sarcoidosis, they did not suffer from severe fatigue and did not cross the mMRC cut-off level for dyspnea. Scores on Physical Activity Level Scale also suggest that the patients were rather physically active. Offering training early in the disease course can perhaps hinder the vicious circle to begin. Moreover, none of the patients was on treatment and treatment itself can cause side-effects affecting quality of life and fatigue. Another explanation to this can be that patients included in the study were consecutively picked from patients fulfilling the inclusion criteria and not because of symptoms.
Major limitations of this study include the lack of a randomized-controlled design, a relatively small study sample, and a rather short observation period. The voluntary nature of inclusion may have influenced the results as motivation and will power can be important factors in achievements. Also, our way of inclusion probably led to a gender imbalance, thus we cannot rule out a different effect of exercise between men and women. At the start of the study, no validated and reliable questionnaires specific for sarcoidosis were available in Swedish to measure fatigue, dyspnea, and health status, which may have led to a bias in the estimation of symptoms and impairments. Moreover, as we only used the measurement of ACE and CRP as surrogate markers for overall disease activity, we are unable to tell if the training affected extrapulmonary disease activity.
Major strengths include good adherence to training and follow-up assessments, the homogeneity of included subjects (treatment-naive, newly diagnosed), as well as exploration of the lung inflammation with bronchoscopy before and after the training. Moreover, we used a high-intensity protocol, whereas other studies have used loads at patient´s preference [Citation21] and low-intensity training [Citation7].
Conclusions
A 12-week high-intensity resistance training protocol combined with inspiratory muscle training at regular intensity in treatment-naive, newly diagnosed sarcoidosis patients led to a significant increase in muscular strength, was well tolerated and no sign of worsening disease was detected, rather some data point towards a decreased inflammation. Thus, high-intensity resistance training seems to be a safe and attractive intervention in patients with sarcoidosis. The results provide a basis for larger randomized trials, which could be achieved by including more centers. Assessment of extrapulmonary disease activities should also be considered in future studies.
Declaration of interest statement
The authors report no conflicts of interest.
Acknowledgments
The authors thank research nurses Heléne Blomqvist, Margitha Dahl, Gunnel de Forest, and Emma Sundström; outpatient clinic nurses Nina Almberg, Carol Parra Troncoso, and Marie Webrink Persson; biomedical analyst Benita Dahlberg; physiotherapists Maria Nyqvist and Ulrika Thunström.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Notes on contributors
Susanna Kullberg
Susanna Kullberg is a senior consultant. She has a special interest in sarcoidosis and combines clinical work with translational sarcoidosis research.
Natalia V Rivera
Natalia V Rivera is an assistant professor. She has an extensive experience in genetics and molecular mechanisms of sarcoidosis, as well as statistical methods.
Maria J Eriksson
Maria J Eriksson is an associate professor and senior consultant. She has a broad knowledge on how to use different methods for assessing cardiac and pulmonary function, both in daily clinical practice and research.
Johan Grunewald
Johan Grunewald is a professor. He leads a dynamic research group focusing especially on immunology in sarcoidosis and has published several papers in this field.
Anders Eklund
Anders Eklund is a senior professor. He initiated extensive research on interstitial lung disorders with focus on sarcoidosis and has published many papers in this field.
References
- Hunninghake GW, Costabel U, Ando M, et al. ATS/ERS/WASOG statement on sarcoidosis. American thoracic society/European respiratory society/world association of sarcoidosis and other granulomatous disorders. Sarcoidosis Vasc Diffuse Lung Dis. 1999;16(2):149–11.
- Grunewald J, Brynedal B, Darlington P, et al. Different HLA-DRB1 allele distributions in distinct clinical subgroups of sarcoidosis patients. Respir Res. 2010;11:25.
- Drent M, Mansour K, Linssen C. Bronchoalveolar lavage in sarcoidosis. Semin Respir Crit Care Med. 2007;28(5):486–495.
- Costabel U. CD4/CD8 ratios in bronchoalveolar lavage fluid: of value for diagnosing sarcoidosis? Eur Respir J. 1997;10(12):2699–2700.
- Marcellis RG, Lenssen AF, Elfferich MD, et al. Exercise capacity, muscle strength and fatigue in sarcoidosis. Eur Respir J. 2011;38(3):628–634.
- De Vries J, Rothkrantz-Kos S, van Dieijen-visser MP, et al. The relationship between fatigue and clinical parameters in pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2004;21(2):127–136.
- Marcellis R, Van der Veeke M, Mesters I, et al. Does physical training reduce fatigue in sarcoidosis? Sarcoidosis Vasc Diffuse Lung Dis. 2015;32(1):53–62.
- Spruit MA, Thomeer MJ, Gosselink R, et al. Skeletal muscle weakness in patients with sarcoidosis and its relationship with exercise intolerance and reduced health status. Thorax. 2005;60(1):32–38.
- Karadalli MN, Bosnak-Guclu M, Camcioglu B, et al. Effects of inspiratory muscle training in subjects with sarcoidosis: a randomized controlled clinical trial. Respir Care. 2016;61(4):483–494.
- Marcellis RG, Lenssen AF, de Vries J, et al. Reduced muscle strength, exercise intolerance and disabling symptoms in sarcoidosis. Curr Opin Pulm Med. 2013;19(5):524–530.
- Wirnsberger RM, Drent M, Hekelaar N, et al. Relationship between respiratory muscle function and quality of life in sarcoidosis. Eur Respir J. 1997;10(7):1450–1455.
- Kabitz HJ, Lang F, Walterspacher S, et al. Impact of impaired inspiratory muscle strength on dyspnea and walking capacity in sarcoidosis. Chest. 2006;130(5):1496–1502.
- Baydur A, Alsalek M, Louie SG, et al. Respiratory muscle strength, lung function, and dyspnea in patients with sarcoidosis. Chest. 2001;120(1):102–108.
- Brancaleone P, Perez T, Robin S, et al. Clinical impact of inspiratory muscle impairment in sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2004;21(3):219–227.
- Cho PSP, Vasudevan S, Maddocks M, et al. Physical inactivity in pulmonary sarcoidosis. Lung. 2019;197:285–293.
- Dowman L, Hill CJ, Holland AE. Pulmonary rehabilitation for interstitial lung disease. Cochrane Database Syst Rev. 2014;10:Cd006322.
- White LJ, Castellano V, Mc Coy SC. Cytokine responses to resistance training in people with multiple sclerosis. J Sports Sci. 2006;24(8):911–914.
- Kierkegaard M, Lundberg IE, Olsson T, et al. High-intensity resistance training in multiple sclerosis - An exploratory study of effects on immune markers in blood and cerebrospinal fluid, and on mood, fatigue, health-related quality of life, muscle strength, walking and cognition. J Neurol Sci. 2016;362:251–257.
- Loprinzi PD, Walker JF, Lee H. Association between physical activity and inflammatory markers among U.S. adults with chronic obstructive pulmonary disease. Am J Health Promot. 2014;29(2):81–88.
- Lundberg IE, Nader GA. Molecular effects of exercise in patients with inflammatory rheumatic disease. Nat Clin Pract Rheumatol. 2008;4(11):597–604.
- Strookappe B, Swigris J, De Vries J, et al. Benefits of physical training in sarcoidosis. Lung. 2015;193(5):701–708.
- Naz I, Ozalevli S, Ozkan S, et al. Efficacy of a structured exercise program for improving functional capacity and quality of life in patients with stage 3 and 4 sarcoidosis: a randomized controlled trial. J Cardiopulm Rehabil Prev. 2018;38(2):124–130.
- Strookappe B, Saketkoo LA, Elfferich M, et al. Physical activity and training in sarcoidosis: review and experience-based recommendations. Expert Rev Respir Med. 2016;10(10):1057–1068.
- Judson MA, Costabel U, Drent M, et al. The WASOG sarcoidosis organ assessment instrument: an update of a previous clinical tool. Sarcoidosis Vasc Diffuse Lung Dis. 2014;31(1):19–27.
- Olsen HH, Grunewald J, Tornling G, et al. Bronchoalveolar lavage results are independent of season, age, gender and collection site. PLoS One. 2012;7(8):e43644.
- Jones PW. Interpreting thresholds for a clinically significant change in health status in asthma and COPD. Eur Respir J. 2002;19(3):398–404.
- Valko PO, Bassetti CL, Bloch KE, et al. Validation of the fatigue severity scale in a Swiss cohort. Sleep. 2008;31(11):1601–1607.
- Grimby G, Frandin K. On the use of a six-level scale for physical activity. Scand J Med Sci Sports. 2018;28(3):819–825.
- Mahler DA, Wells CK. Evaluation of clinical methods for rating dyspnea. Chest. 1988;93(3):580–586.
- American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Med Sci Sports Exerc. 2009;41(3):687–708.
- Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14(5):377–381.
- Berlin M, Fogdell-Hahn A, Olerup O, et al. HLA-DR predicts the prognosis in Scandinavian patients with pulmonary sarcoidosis. Am J Respir Crit Care Med. 1997;156(5):1601–1605.
- Strookappe B, Elfferich M, Swigris J, et al. Benefits of physical training in patients with idiopathic or end-stage sarcoidosis-related pulmonary fibrosis: a pilot study. Sarcoidosis Vasc Diffuse Lung Dis. 2015;32(1):43–52.
- Planck A, Eklund A, Grunewald J. Markers of activity in clinically recovered human leukocyte antigen-DR17-positive sarcoidosis patients. Eur Respir J. 2003;21(1):52–57.
- Asimakos A, Toumpanakis D, Karatza MH, et al. Immune cell response to strenuous resistive breathing: comparison with whole body exercise and the effects of antioxidants. Int J Chron Obstruct Pulmon Dis. 2018;13:529–545.
- Aquino-Junior JCJ, MacKenzie B, Almeida-Oliveira AR, et al. Aerobic exercise inhibits obesity-induced respiratory phenotype. Cytokine. 2018;104:46–52.
- Fernandes P, de Mendonca Oliveira L, Bruggemann TR, et al. Physical exercise induces immunoregulation of TREG, M2, and pDCs in a lung allergic inflammation model. Front Immunol. 2019;10:854.
- Charususin N, Gosselink R, Decramer M, et al. Randomised controlled trial of adjunctive inspiratory muscle training for patients with COPD. Thorax. 2018;73(10):942–950.
- Gosselink R, De Vos J, van den Heuvel SP, et al. Impact of inspiratory muscle training in patients with COPD: what is the evidence? Eur Respir J. 2011;37(2):416–425.
- Geddes EL, O’Brien K, Reid WD, et al. Inspiratory muscle training in adults with chronic obstructive pulmonary disease: an update of a systematic review. Respir Med. 2008;102(12):1715–1729.
- Vainshelboim B, Oliveira J, Yehoshua L, et al. Exercise training-based pulmonary rehabilitation program is clinically beneficial for idiopathic pulmonary fibrosis. Respiration. 2014;88(5):378–388.
- Naz I, Sahi H, Uçsular FD, et al. A comparison trial of eight weeks versus twelve weeks of exercise program in interstitial lung diseases. Sarcoidosis Vasculitis and Diffuse Lung DISEASES. 2018;35(4):299–307.
Appendix A.
Details on BAL procedure. Portions = number of installed aliquots and volume (ml) in every aliquot. Recovery = percentage of installed volume that was retrieved; nd = not determined.
Appendix B.
Appendix C.
Appendix D.
Appendix E.
Additional information on study subjects
Appendices B–D. Adherence to assessment. ACEI = patient was treated with angiotensin converting enzyme inhibitor, Training sessions = number of completed sessions out of 24, X = assessment performed, 0 = assessment not performed, numbers 1,2 and 3 denotes before training, first follow-up and second follow-up respectively. Patient number 4 refused to undergo the second bronchoscopy.
Patient number 3 might have had an LS a few years earlier according to symptoms he described but that could not be certified, no diagnostic procedures were performed at that time.
Patient number 9 had pain due to arthrosis in his right knee when training started and was therefore excluded from leg press at initial testing. The pain disappeared during the training period. Patient number 1 got herpes zoster, patient number 4 the flu, patient number 8 an upper airway infection, and patient number 10 pertussis. Training was stopped but continued after recovery. Patient number 8 had a history of spinal disc herniation and had occasionally pain in his back. During the training, also pain in his right knee emerged. MRI disclosed an old meniscus rupture. He was sent to an orthopedic who regarded this as a chronic condition and not related to the training. In the end of the training period, he also got pain in both elbows, pain increased in biceps curls and abdomen flexion (the elbows were then pressed against a cushion), subsequently, these exercises were not performed at follow-up. The cause for this was not revealed, but the symptoms disappeared after cessation of training.
At first follow-up, patient number 1 disclosed a decrease of chest X-ray infiltrates and number 3, a progress with increasing dyspnea, chest X-ray infiltrates and a deteriorating lung function, e.g. FVC decreased from 96 to 82% of predicted. This patient was put on systemic treatment with corticosteroids (30 mg prednisolone initially).
At second follow-up, patient number 8 disclosed a regress and number 2, 7, and 9 a minor progress. The progress was characterized by a slight increase of chest X-ray infiltrates but no one of the patients experienced worsening of respiratory symptoms, lung function parameters remained about the same and they were not in need of treatment.
Both patients with a regress (1 and 8) disclosed a slight decrease of chest X-ray infiltrates but lung function parameters remained about the same and the patients did not report any major changes in symptoms.