ABSTRACT
Background: Chronic obstructive pulmonary disease (COPD) is a common comorbidity in patients with lung and head- and neck cancer. Patients with lung cancer who also suffer from COPD have a worse prognosis than patients with lung cancer and no COPD. It has previously been shown that diagnosis and treatment of concomitant COPD in patients with newly diagnosed lung- or head and neck cancer need optimization. In this randomized, controlled trial we aimed to assess if intervention directed at improving treatment for COPD in these patients improved health-related quality of life (QoL).
Methods: During 2014, we randomized 114 patients referred for oncological treatment at a large university hospital in the Capital Region of Denmark, to either usual care or intervention regarding concomitant COPD. The intervention consisted of two visits in an out-patient clinic established at the oncological department and staffed with a pulmonary physician. At baseline, week 13 and week 25, all patients filled out the cancer- and COPD-specific QoL questionnaires CAT and EORTC, respectively. The primary outcome was change in CAT-score between control- and intervention group. The secondary outcome was change in EORTC.
Results: There was no change in CAT-score by week 13 or 25 between the groups. For the EORTC there was a statistically significant improvement only in the fatigue domain at week 13 (p = 0.03), but not at week 25. There was a trend towards less dyspnea in the intervention group at week 13, measured by EORTC (p = 0.07). Mortality by week 25 was similar in both groups.
Conclusion: In this population of severely ill cancer patients, we did not find that this intervention, focusing on inhaled COPD medication, for the management of COPD had any convincing positive impact on the patients’ perceived quality of life compared with usual care. Further studies are needed.
KEYWORDS:
Introduction
Breathlessness, fatigue, and cough/sputum are the most common symptoms in COPD [Citation1,Citation2,] and are also prevalent in lung cancer (LC) [Citation3]. Patients with head- and neck cancer (HNC) suffer from fatigue and cough and to some degree also dyspnea [Citation4,Citation5,]. Smoking is a known risk factor for both LC and HNC, thus making COPD a common comorbidity in both diseases [Citation6,Citation7,].
Studies have shown a negative correlation between a number of comorbidities, including COPD, and survival of both HNC and LC [Citation8,Citation9,]. A recent study from Norway comprising 174 patients newly diagnosed with LC found that the prevalence of concomitant COPD or emphysema was 69% [Citation10]. Among the 212 patients with either LC or HNC examined for inclusion in the present study, the prevalence of COPD was 54% (69% of those with LC and 25% with HNC) [Citation11].
COPD is widely underdiagnosed and under-treated and it is estimated that there are between 3- and 400.000 patients with COPD in Denmark, of which approximately 100.000 receive medical treatment [Citation12]. Smoking cessation is the most powerful intervention for changing disease progression and prognosis, however, symptomatic patients will normally require treatment with bronchodilators. Pharmacotherapy for COPD is used to reduce symptoms, reduce frequency and severity of exacerbations and improve health status and exercise tolerance. The choice of medication depends on severity of symptoms, degree of airflow limitation and risk of exacerbations [Citation1].
There is very limited knowledge about the possible effects of optimizing the treatment of COPD in patients with smoking-related cancers. The presence of COPD has in some studies been shown to worsen the prognosis of non-small cell lung cancer (NSCLC) but the results are not consistent [Citation8,Citation9,]. We have previously shown that patients with LC and HNC are also underdiagnosed and/or undertreated for COPD and that it is feasible to introduce a pulmonary clinic in an oncological outpatient setting [Citation11]. Regardless of the intention of the cancer treatment (curative or palliative), optimizing the management of COPD is likely to improve patient quality of life due to the fact that treatment with bronchodilators reduces dyspnea and frequency of exacerbations [Citation13]. Therefore, the objective of the present study was to test the hypothesis that diagnosis and follow-up of patients with COPD on a regular basis in a pulmonary outpatient clinic staffed by a pulmonary physician, established in the oncological department, can reduce respiratory symptoms and fatigue and improve QoL in patients with LC or HNC referred for oncological treatment.
Material and methods
This RCT study was an investigator-initiated study.
Setting
A COPD outpatient clinic was established on 1 February 2014, at the Unit of Thoracic and Head and Neck Oncology, Herlev University Hospital and was staffed by a pulmonologist.
All newly diagnosed HNC and LC patients referred to the Department of Oncology, Herlev Hospital was screened for COPD by spirometry, and if present, invited to participate in a randomized trial. Patients were randomized to either continuous follow-up by pulmonologist for 24 weeks (intervention) or management as previously (control). All patients provided written informed consent. The study including randomization procedure, inclusion criteria, and sample size calculation has been described in detail previously [Citation11].
The trial was designed as a non-blinded, controlled, randomized trial. The inclusion ran to 31 December 2014. Patients in the intervention group were offered treatment of COPD according to Global Obstructive Lung Disease (GOLD) guidelines 2014 [Citation14] and were scheduled for two control appointments at 12 and 24 weeks, respectively, where adjustment of the COPD treatment was considered. Patients in the control group were to continue their current, if any, treatment and follow-up of COPD. At first visit, the patients filled out the following QoL-questionnaires: European Organisation for Research and Treatment of Cancer (EORTC) (Q30 + LC13) [Citation15,Citation16,] and COPD Assessment Test (CAT) [Citation17]. At week 13 and week 25 CAT and EORTC questionnaires were sent to both intervention- and control patients.
The study was approved by the local ethical committee (capital region of Denmark), H-3-2013-174. Unfortunately, we did not register the study at trials.gov. or another registration site.
Participants
All newly diagnosed patients with lung and head/neck cancer referred to the Department of Oncology at Herlev Hospital were screened for COPD with pre- and post-bronchodilator spirometry. The only patients not entering the screening phase of the study were those who were planned to have now or short duration of treatment (less than 1 month). Patients were seen at the pulmonary clinic within the first 2 weeks after referral. The LC patients included all primary lung cancers, and the HNC patients included primary cancers in larynx, hypopharynx, oral cavity, pharynx, and oropharynx. Patients treated with a curative as well as palliative intent were eligible. At the first visit to the pulmonary clinic, spirometry was performed, regardless of a prior COPD diagnosis. Reversibility testing was done unless the patient had used a beta-2-agonist the same day, in order to exclude patients with undiagnosed asthma. Patients with an FEV1/FVC ratio <0.70, no significant beta-2-reversibility and no actual or previous doctor-diagnosed asthma were eligible for the randomized trial and were invited to participate.
After inclusion, patients were randomized to either intervention or control, and medical history and baseline information was obtained. Based on spirometry, exacerbation history, MRC-grade and CAT-score, whichever score was equal to or above the thresholds of 3 and 10, respectively, the GOLD group was determined. The pulmonologist assessed whether the current COPD medication was in accordance with the GOLD guidelines.
Intervention group
In the intervention group, the pulmonary physician used the GOLD group classification to assess if there was a need for changes in COPD medication. Choice of drug and device was done in dialogue with the patients. Patients were asked to demonstrate the inhalation technique using a placebo device to make sure that the medication was taken correctly, and inspiratory capacity was measured with the In-Check-Dial. This was also done with the patients’ current device if no changes to medication were made. Patients who were classified as GOLD group A would in most cases not need any COPD medication. All patients were scheduled for a second visit to the pulmonary clinic after 12 weeks and a third visit after 24 weeks. If patients had only few respiratory symptoms and no medication was prescribed, a telephone appointment rather than physical appearance was arranged. All patients were instructed to contact the clinic between visits if they had questions regarding the COPD medication. The clinic was only open once a week; hence, any acute respiratory problems would have to be taken care of elsewhere. At the follow-up visits, any need for changes in the COPD medication was assessed by the physician and discussed with the patient. Also, inhalation technique was checked again. If patients did not show up for a scheduled visit, the physician would call the patient and arrange for a new visit.
Control group
For the patients in the control group, no changes in the medication were suggested, but they were encouraged to continue any planned follow-up for COPD or make an appointment with their general practitioner in case of a new COPD diagnosis. All patients were informed of the COPD diagnosis found at screening. Changes in COPD medication after inclusion were not reported.
Follow-up
The follow-up period was 25 weeks. CAT and EORTC questionnaires were sent to patients in both the intervention group and control group at weeks 13 and 25. The patients in the intervention group thus received the questionnaires approximately 10 days after the visits to the pulmonary clinic. Should the questionnaires not be returned within 1 week, the pulmonary physician would remind the patient by phone. If the questionnaires were not returned after 4 weeks, the patient was considered lost to follow=up. The last control visit was in June 2015.
Randomization
Randomization was performed 1:1 by computer using ARRACT software and was stratified to ensure equal distribution of sex, age, LC, and HNC between the control group and the intervention group.
Outcomes
The primary endpoint was mean difference in change in CAT-score over time from baseline to 13 weeks and baseline to 25 weeks between the control group and the intervention group. Secondary endpoints included QoL as assessed by EORTC30 questionnaire, which is a validated QoL-questionnaire for cancer patients and the LC13, which is a specific supplementary questionnaire for patients with lung cancer. The LC13 questionnaire was also used for the HNC patients since it covers symptoms associated with COPD. The questionnaires consist of both single item scales and multi-item scales. The EORTC30 is divided into global health scales, functioning scales and symptom scales. The LC13 questionnaire only consists of symptom scales. A higher score in global health status and a functioning score mean better health and higher level of functioning. A higher score in a symptom scale means higher symptom burden. Since the intervention was expected to relieve only respiratory symptoms and thereby global health/functioning the following scales were chosen for analysis: EORTC30: global health status, physical functioning, role functioning, fatigue, and dyspnea. LC13: dyspnea and coughing. A change in functioning scales and symptom scales of 10 points is regarded as clinically relevant [Citation18].
CAT is an eight-question questionnaire on a VAS-scale covering the most frequent symptoms of COPD and validated to determine disease severity. The score range is between 0 and 40, and a score >10 indicates a high symptom burden. A change of 2–3 points is regarded as clinically relevant [Citation19,Citation20,].
Statistical methods
Continuous variables are presented as mean with standard deviation. For categorical variables, frequency counts and percentages are presented as summary statistics for the subgroups of interest. Univariate analysis was performed using chi-squared test for categorical data and independent t-test for continuous variables. SPSS 21 was used to analyze data. Power calculations showed that either 36, 51 or 60 patients were needed in each group to show a significant difference (MCID) in CAT-score after the intervention of 2, 2.5 and 3 points, respectively. This is based on a superiority design, assuming a power (beta) of 80%, significance level (alpha) of 5%, standard deviation (SD) 4.5 points. The SD value was chosen arbitrarily since we had no previous data on the dispersion of CAT scores in a similar population.
Results
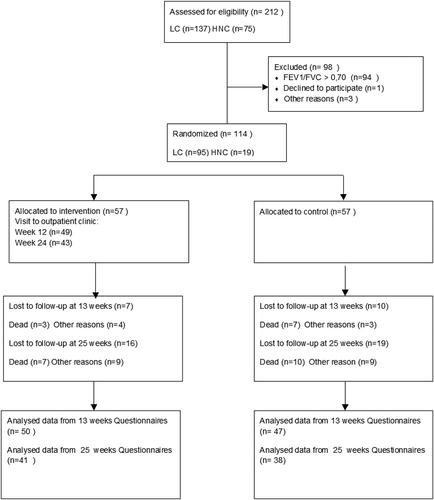
shows a Consort flowchart of inclusion and follow-up. From 01.02.2014 to 31.12.2014, a total of 212 patients were assessed in the pulmonary outpatient clinic for inclusion in the trial. Out of these 137 (65%) had LC and 75 (35%) had HNC. One hundred and fourteen patients with COPD were included in the trial, LC n = 95 (83%) and HNC n = 19 (17%), and 57 patients randomized to each group.
Of 57 patients in the intervention group, 49 (86%) returned for the week 12 visit to the pulmonary clinic. Two patients did not attend the week 12 visit but returned the questionnaires. One patient came for the week 12 visit but died before returning questionnaires. Thus, 50 week 13 questionnaires were available for analysis. For the scheduled week 24 visit, 43 patients attended the clinic or had a telephone consultation. Two patients did not attend the clinic but returned questionnaires. One had died and three failed to return the questionnaires for unknown reasons. A total of 41 week 25 questionnaires were available for analysis in the intervention group. In the control group 47 week 13 and 38 week 25 questionnaires were available for analysis.
Most of the patients who did not return the QoL-questionnaires could not be reached and thus gave no reason. Nine patients from the intervention group stated that they did not feel the need for neither any COPD medication nor visits to the clinic.
During the trial period, there was no significant difference in mortality between the intervention group (dead n = 7, 12.3%) and the control group (dead = 10, 17%), p = 0.43.
shows the baseline characteristics of the randomized patients including performance status, cancer type and stage, and comorbidity. Distribution between the usual care group and the intervention group was skewed towards a higher number of patients with mild COPD (GOLD A) in the control group and more women in the intervention group.
Table 1. Baseline characteristics of the patients. Figures are presented as mean (SD) or numbers (%)
shows mean scores in EORTC30, LC13, and CAT at baseline. In general, there was an equal distribution in scores between the groups. However, the usual care group scored higher in pain than the intervention group (30.9 vs. 19.9). The intervention group scored higher in dyspnea in EORTC30 (37.5 vs. 27.5); however, there was no relevant difference in dyspnea when measured in LC13. LC13-dyspnea is a three-item scale compared to EORTC30 – dyspnea, which is a single item scale.
Table 2. QoL-questionnaires at baseline. Figures are presented as mean (SD)
shows the differences in changes in mean scores between baseline scores and week 13 scores and week 25 scores, respectively, for the intervention group (I) and the usual care group (UC). For the difference between groups over time, a positive number means that the intervention group scores changed in a more positive direction than differences in scores for the usual care group. For the symptom scales and CAT score, a lower figure indicates improvement.
Table 3. Change in relevant scales/items on EORTC 30, LC13, and CAT from baseline to 13 and 25 weeks follow up. Figures are presented as mean (SD)
No difference between the intervention and control group was found in CAT scores either at week 13 or week 25. For EORTC, there were no clinically relevant or significant differences between the groups over time in global health status or functioning scales at either week 13 or week 25. However, in symptom scales at week 13, there was a significant improvement in the change in fatigue (−12.4 points, p = 0.03) in favor of the intervention. This difference remained numerically unchanged but was no longer significant at week 25 (−12.3, p = 0.07). At week 13 there was a clinically relevant but not significant difference in EORTC dyspnea (−11.2, p = 0.07) favoring the intervention. However, no difference between groups was found when the three-item scale LC13 was used (2.7, p = 0.5), and at week 25 no differences in either EORTC30-dyspnea (−2.7, p = 0.7) or LC13-dyspnea (0.3, p = 1.0) were found. Multivariate analysis was also done but no new clinically relevant or significant differences in mean score differences emerged (data not shown).
Discussion
The overall result of our study is that this setup with increasing focus on diagnosis and correct treatment with inhalation medication for COPD does not attenuate self-rated symptom burden or increase QoL in patients with co-existing COPD and lung cancer. The only significant result was EORTC Fatigue at 13 weeks which probably is a chance finding since the same result was not found in the CAT scores. The study was powered for finding a difference in CAT score and not the EORTC. Only approximately one third of the patients were diagnosed with COPD before inclusion and of these only around 50% received the correct inhalation medication according to GOLD. Unfortunately, the distribution of patients in GOLD A-D was skewed towards more patients in the control group being in group A compared to the intervention group (45% vs. 30%). This would, however, have made a positive finding likelier.
A limitation of this study is that the statistical power was limited due to small sample size. The SD chosen for the power calculation (4.5) is slightly lower than found in intervention studies of COPD [Citation21]. With a larger study, the ability to detect significant differences would increase. There is also a risk that an underpowered study may not detect real differences due to change variation. However, the trend in our observations does not indicate an effect of the intervention. For example, the CAT score actually worsened in the intervention group, while is slightly improved in the control group (CAT scores +0.8 and −0.4, respectively). Unexpectedly the baseline CAT score was only approximately 13 in both groups, which also makes it more difficult to detect improvements from an intervention. In future studies, careful consideration of study size (e.g. including more study sites), type of intervention and outcome (e.g. a composite QoL endpoint) is warranted.
It is highly possible that the follow-up period of 25 weeks was not long enough for detecting a true difference. The relatively large loss to follow-up is also of concern, since this can possibly introduce an information bias, meaning that those who are most ill (or most well) are also most likely not to return the schedules or show up for an appointment. The reasons for this loss to follow-up could possibly be skewed between the arms. Another likely explanation for the negative result could be that both the symptomatology and the focus of attention in the current context are driven by the cancer and not the COPD. A recent study from Spain, very similar to ours but retrospective, found a 29% prevalence of COPD in their cohort of LC patients and also that 60% were undiagnosed prior to the LC diagnosis [Citation22]. Not unexpectedly, those with a known COPD diagnosis had more severe airway obstruction and COPD-related symptoms, whereas the LC was more progressed in patients with newly diagnosed COPD. Nevertheless, even if this is the primary explanation for this negative result, the treatment of COPD has since 2014 and to date increased the focus on dual bronchodilation. This continuous development in treatment of COPD might have influenced the results. We do not have data on how many of the patients were treated with only one bronchodilator and/or inhaled corticosteroids (ICS) in the two groups, respectively. A very recent observational Swedish study of risk factors for LC in a large cohort of patients with COPD found that a diagnosis of concomitant asthma and prescription of ICS was independent predictors of a decreased risk of developing LC [Citation23]. The study was, however, limited by no information of pack years of smoking and no recent spirometry for classifying the severity of COPD. We do not have any data on adherence to prescribed medication for COPD in the present study. This is a key issue in the treatment of COPD, where adherence is known to be low in general [Citation24]. It is possible that patients in the control group, who underwent a spirometry and were either diagnosed with COPD or reminded of this disease had a medical follow-up outside this randomized setting. In this case, it would dilute any effect of our intervention. Moreover, patients in the control group could possibly already be treated optimally by their general practitioner.
Another limitation is that the pulmonary outpatient clinic did not offer any form of pulmonary rehabilitation, which may be a central reason for the negative result. Pulmonary rehabilitation has been shown to be the most effective therapeutic strategy to improve symptoms and health status [Citation25]. Furthermore, we do not have data on medication adherence which also may influence the negative result. At the start of an oncological treatment at lot of new information is given, that probably weakens the patients’ focus on new inhalers. Finally, we did not have any smoking cessation intervention as part of our study and the possible continued tobacco smoking may cause continued symptoms.
The massive symptom burden of lung cancer alone makes it difficult to improve QoL and optimizing medication for concomitant COPD, i.e, for dyspnea relief may rather have to be a part of a palliative approach. Specialized palliative care has been shown to improve QoL for patients with lung cancer [Citation26], hence focusing almost solely on inhalation medication might not be enough to improve self-rated QoL or symptom burden for patients with both COPD and lung cancer. With this in mind, we still believe that it is not worthless to diagnose and address COPD in patients with newly diagnosed lung cancer.
Conclusion
In conclusion, the present study indicates that intervention focusing almost exclusively on optimizing inhaled medication for COPD patients with co-existent lung cancer does not improve symptom control and QoL as assessed by the CAT-questionnaire. We conclude that further, larger studies are needed to improve care of patients with COPD and lung cancer.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Notes on contributors
Magnus Gottlieb
Magnus Gottlieb is a specialty registrar in Respiratory Medicine in the Capital region of Denmark and has previously published results from the present study and also on monitoring of COPD.
Anders Mellemgaard
Anders Mellemgaard is consultant, ph.d. in Oncology, formerly employed at Herlev University Hospital in the Capital region of Denmark, now Bornholms Hospital. He is especially interested in Respiratory Oncology and related quality of life issues.
Kristoffer Marsaa
Kristoffer Marsaa is a consultant in Respiratory Medicine and employed at the Palliative Unit at Herlev University Hospital in the Capital region of Denmark. His main interest in research concerns palliation and advance care planning in COPD.
Nina Godtfredsen
Nina Godtfredsen is consultant, ph.d. in Respiratory Medicine at Hvidovre University Hospital in the Capital region of Denmark and also associate research professor and lecturer at Institute of Clinical Medicine, University of Copenhagen. Her research focus is epidemiology, rehabilitation, comorbidities and smoking cessation in COPD.
References
- [ cited 2019 Apr 4]. Available from: https://goldcopd.org/.
- Miravitlles M, Ribera A. Understanding the impact of symptoms on the burden of COPD. Respir Res. 2017;18(1):67.
- Lim RB. End-of-life care in patients with advanced lung cancer. Ther Adv Respir Dis. 2016;10(5):455–8.
- Mason H, DeRubeis MB, Burke N, et al. Symptom management during and after treatment with concurrent chemoradiotherapy for oropharyngeal cancer: A review of the literature and areas for future research. World J Clin Oncol. 2016;7(2):220–226.
- Chaukar DA, Walvekar RR, Das AK, et al. Quality of life in head and neck cancer survivors: a cross-sectional survey. Am J Otolaryngol. 2009;30(3):176–180.
- Eytan DF, Blackford AL, Eisele DW, et al. Prevalence of comorbidities among older head and neck cancer survivors in the USA. Otolaryngol Head Neck Surg. 2019;160(1):85–92.
- Grose D, Morrison DS, Devereux G, et al. Comorbidities in lung cancer: prevalence, severity and links with socioeconomic status and treatment. Postgrad Med J. 2014;90:305–310.
- Dai J, Liu M, Swensen SJ, et al. Regional emphysema score predicting overall survival, quality of life, and pulmonary function recovery in early-stage lung cancer patients. J Thorac Oncol. 2017;12(5):824–832.
- Sylvester MJ, Marchiano E, Park RC, et al. Impact of chronic obstructive pulmonary disease on patients undergoing laryngectomy for laryngeal cancer. Laryngoscope. 2017;127(2):417–423.
- Ytterstad E, Moe PC, Hjalmarsen A. COPD in primary lung cancer patients: prevalence and mortality. Int J Chron Obstruct Pulmon Dis. 2016;11:625–636.
- Gottlieb M, Marsaa K, Godtfredsen NS, et al. Prevalence and management of pulmonary comorbidity in patients with lung and head and neck cancer. Acta Oncol. 2015;54(5):767–771.
- Hansen JG, Pedersen L, Overvad K, et al. The Prevalence of chronic obstructive pulmonary disease among Danes aged 45–84 years: population-based study. COPD. 2008;5(6):347–352.
- Riley CM, Sciurba FC. Diagnosis and outpatient management of chronic obstructive pulmonary disease: a review. JAMA. 2019;321(8):786–797.
- Vestbo J, Hurd SS, Agustí AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347–365.
- Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–376.
- Bergman B, Aaronson NK, Ahmedzai S, et al. The EORTC QLQ-LC13: a modular supplement to the EORTC Core Quality of Life Questionnaire (QLQ-C30) for use in lung cancer clinical trials. EORTC Study Group on Quality of Life. Eur J Cancer. 1994;30A(5):635–642.
- Jones PW, Harding G, Berry P, et al. Development and first validation of the COPD Assessment Test. Eur Respir J. 2009;34(3):648–654.
- Maringwa JT, Quinten C, King M, et al. Minimal important differences for interpreting health-related quality of life scores from the EORTC QLQ-C30 in lung cancer patients participating in randomized controlled trials. Support Care Cancer. 2011;19(11):1753–1760.
- Kon SS, Canavan JL, Jones SE, et al. Minimum clinically important difference for the COPD Assessment Test: a prospective analysis. Lancet Respir Med. 2014;2(3):195–203.
- Smid DE, Franssen FM, Houben-Wilke S, et al. Responsiveness and MCID Estimates for CAT, CCQ, and HADS in patients with COPD undergoing pulmonary rehabilitation: a prospective analysis. J Am Med Dir Assoc. 2017;18(1):53–58.
- Dodd JW, Hogg L, Nolan J, et al. The COPD assessment test (CAT): response to pulmonary rehabilitation. A multicentre prospective study. Thorax. 2011;66(5):425–429.
- Parrón Collar D, Pazos Guerra M, Rodriguez P, et al. COPD is underdiagnosed in patients with lung cancer: results from the RECOIL study (retrospective study of COPD infradiagnosis in lung cancer). Int J Chron Obstruct Pulmon Dis. 2017;12:1033–1038.
- Sandelin M, Mindus S, Thuresson M, et al. Factors associated with lung cancer in COPD patients. Int J Chron Obstruct Pulmon Dis. 2018;13:1833–1839.
- Rogliani P, Ora J, Puxeddu E, et al. Adherence to COPD treatment: myth and reality. Respir Med. 2017;129:117–123.
- McCarthy B, Casey D, Devane D, et al. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015;(2):CD003793.
- Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363(8):733–742.