ABSTRACT
Radiological presentation of bronchiectasis should prompt the respiratory physician to investigate various differential diagnosis leading to this condition. This case report describes a young non-smoking woman with HPV11 induced laryngeal Recurrent Respiratory Papillomatosis (RRP) since early childhood, who developed progressive exertional dyspnea. A thorough diagnostic process revealed HPV11 infection in the lung parenchyma consistent with RRP in the lower airways, an HPV infection that was most likely obtained from the patient´s mother during vaginal birth.
This case report illustrates that also respiratory physicians should keep RRP in mind in persons with the radiological presentation of bronchiectasis previously diagnosed RRP in the upper airways.
Introduction
Recurrent Respiratory Papillomatosis (RRP) is a rare disease characterized by recurrent growth of papillomas primarily in the larynx and occasionally in other parts of the aerodigestive tract [Citation1,Citation2]. Although RRP is a benign disease, malignant transformation to squamous cell carcinoma has been described [Citation2–Citation5]. RRP is caused by Human Papilloma Virus (HPV) subtypes 6 or 11, of which subtype 11 more often leads to severe affection of the larynx, trachea and bronchi and malignant transformation [Citation2,Citation3]. There is currently no curative treatment. Available epidemiological data on RRP are sparse, with no reported data on prevalence and with estimated incidence proportions of 2 per 100,000 in adults and 4 per 100,000 in children, respectively [Citation2,Citation6]. Approximately 1–4% of patients with RRP develop pulmonary papillomatosis [Citation1,Citation3,Citation7].
We describe a young woman with laryngeal RRP since early childhood who in her adolescence developed respiratory symptoms that turned out to be caused by pulmonary parenchymal RRP.
Case presentation
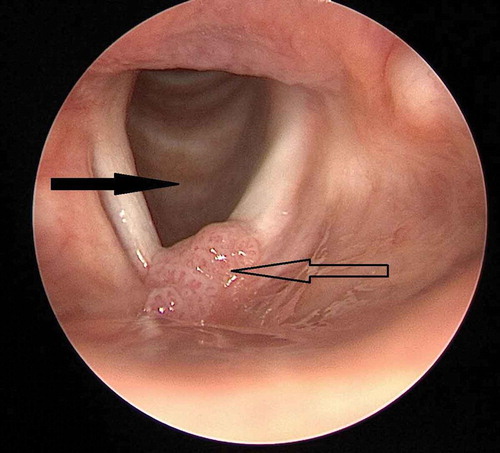
An 18-year-old non-smoking woman was referred to the Department of Respiratory Medicine because of increasing dyspnea. Since the age of 2 years she had gone through more than 40 laser-surgical procedures at the Department of Head and Neck surgery due to laryngeal RRP (). Additionally, she had received adjuvant therapeutic HPV vaccination (Gardasil®, Merck Sharp & Dohme) when she was 17 years old and prior to sexual debut.
Figure 1. Endoscopic view of larynx. Solid arrow: trachea with normal mucosa. Hollow arrow: papillomatosis in the anterior commissure of the vocal cords
The dyspnea had progressed over the past 6 months and at the time of referral she had exercise-induced minor shortness of breath corresponding to a medical council research (MRC) score of 3. In the recent few months, intermittent coughing occurred, but without any concurrent symptoms as palpitations, angina pectoris, rhino-conjunctivitis, pleurisy, sputum production, night sweating or fever and her weight was stable with a body mass index of 25 kg/m2
Investigations
Broad biochemical analyses including alpha 1-antitrypsin in the blood were all within normal ranges. Lung physiological investigations included normal age- and gender-adjusted spirometry- and peak flow values. The diffusion capacity of carbonmonooxid for the lung was however slightly reduced to 74% of predicted. A negative methacholine challenge test (MCT) excluded bronchial hyperresponsiveness and asthma. A chest X-ray revealed bronchial affection with apical peribronchial thickening on the right upper lobe and localized emphysema bullae.
A subsequent high-resolution computed tomography (HRCT) showed several thick-walled, large cysts (up to 3 cm) in relation to terminal bronchi compatible with cystic bronchiectasis with mucus plugging and pneumocele like cysts as potential sequelaes from previous pneumonias ( and ). In order to distinguish other cystic lung diseases from cystic bronchiectasis and also to examine underlying opportunistic infection a bronchoscopy with bronchoalveolar lavage (BAL) was conducted. Flow cytometric analyses of BAL showed an increased cell number of 32x10^6 cells per 100 mL, with a dominance of non-pigmented macrophages of 94%, but no neutrophilia or eosinophilia. Furthermore, no cluster of differentiation 1a (CD1a) positive cells were identified. The flowcytometric findings combined with a low value of vascular endothelial growth factor D (VEGF-D) in serum and a negative folliculin (FLCN) gene test and atypical distribution and morphology of the cysts on the HRCT did not support a suspicion of multiple cystic lung diseases (MLCD) as, e.g., lymphangioleiomyomatosis, Birt-Hogg-Dubé syndrome or pulmonary Langerhans-Cell histiocytosis. Microbiological analyses of BAL showed negative culture and polymerase chain reactions (PCR) for bacteria and fungi including pneumocystis jirovecii, herpes simplex virus type 1 and type 2, herpes zoster virus, and cytomegalovirus. PCR analysis of a protected cytobrush specimen sample from the right lung identified DNA corresponding to HPV11 infection.
Figure 2. Coronal (a), sagittal (b) high resolution computed tomography images showing conglomerates of cystic bronchiectasis in upper lobes and thick-walled and heterogeneous sized cysts in lower lobes. In Figure 2(b) the cystic conglomerates are seen in conjunction with an accompanying and bronchiole with bronchial wall thickening
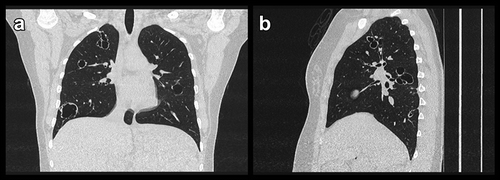
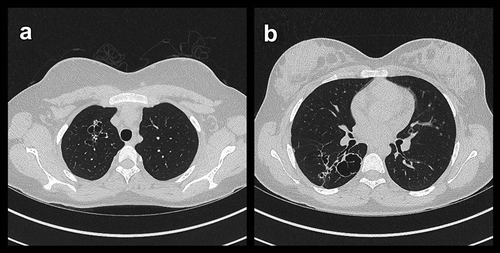
Figure 3. Transversal (A + B) high resolution computed tomography images showing conglomerates of cystic bronchiectasis in upper lobes and thick-walled and heterogeneous sized cysts in lower lobes
The patient’s immunological status was assessed with normal levels of T-and B-lymphocytes, NK cells, and immunoglobulins including immunoglobulin G (IgG) subclasses. However, because of a reduced value of mannose-binding lectin below 50 µg/L and no antibodies to pneumococci, the patient was referred to the Department of Infectious Diseases to assess whether an undiagnosed immune deficiency was present.
Differential diagnosis
The long preceding course of laryngeal RRP prior to the respiratory symptoms obviously led to the suspicion of pulmonary involvement of RRP. The clinical presentation was, however, not unambiguous (i.e. there were no ‘skip lesions’ in trachea and the pulmonary lesions were more cystic than cavitary lesions which are more common in pulmonary RRP). Therefore, it was considered important not only to confirm the suspected diagnosis of pulmonary RRP but also to rule out other possible diagnoses. Differential diagnoses to cystic lung diseases in young adults with symptoms as dyspnea and cough include rare phenotypes of asthma as, e.g., allergic bronchopulmonary aspergillosis (ABPA), emphysema, MLCDs, cystic bronchiectasis, mycobacterial or fungal infection and rarely RRP.
As the patient had a normal spirometry and a negative MCT, asthma and ABPA were excluded, and a normal alfa 1-antitrypsin (A1AD) level in the blood furthermore made A1AD-emphysema implausible.
The majority of the microbiological BAL analyses were negative and cysts/bullae due to underlying mycobacterial or fungal infection were therefore not regarded as a causal explanation. MCLDs were also considered as potential differential diagnoses. However, MCLDs are characterized by multiple intrapulmonary cysts with sharply demarcated thin walls (usually <2–3 mm), which differ from cysts as part of emphysema, cystic bronchiectasis, and honeycombing [Citation8,Citation9]. This patient had thick-walled cysts in relation to dilated and thick-walled bronchi ( and ) which in combination with a negative FLCN gene test, a low value of serum VEGF-D and absence of CD1a-positive cells in BAL made MCLD unlikely. A consensus diagnosis of cystic bronchiectasis was reached at a multidisciplinary discussion among pulmonologists, pathologists, and radiologists.
Treatment
The patient had already gone through multiple CO2-laser surgical procedures for laryngeal RRP at the department of Head and Neck surgery and had received therapeutic HPV vaccine. At present, the patient awaits final decision regarding potential underlying immune deficiency, but is followed with lung physiological assessment every 6 months and annual HRCT in order to monitor disease progression.
Discussion
RRP is a rare disorder and involvement of the lungs is very rare [Citation1–Citation3,Citation7]. Although the disease is benign, it can lead to severe morbidity and mortality due to airway obstruction and malignant transformation to carcinoma in the affected organs [Citation1–Citation3,Citation5].
Clinical symptoms depend on the location and severity of the papillomas. Due to the predilection of RRP lesions for the vocal cords, the most common symptom is voice problems [Citation10–Citation12]. In case of large papillomas in the upper airways, or widespread involvement of the trachea or lower airways, wheezing, coughing, dyspnea and acute respiratory insufficiency may arise, and the disease can therefore be confused with asthma [Citation2].
Surgery with cold instruments, lasers, or microdebriders is the mainstay of treatment in RRP [Citation2,Citation13]. Other methods of treatment are cryotherapy and electrocoagulation. Treatment is targeted at removal of the papillomas, reducing disease spread, creating safe airways, preserving nearby anatomical structures, improving voice quality if necessary and increasing time between surgical procedures [Citation2,Citation13]. In severe cases with aggressive growth of papillomas in the upper airways, tracheotomy may be necessary to secure a safe airway [Citation2,Citation10,Citation13].
To improve the surgical outcome and prolong the symptom-free intervals, numerous adjuvant therapeutic modalities have been applied, but adjuvant therapy is only used in a minority of patients with RRP [Citation2]. Adjuvant medical therapy contains antiviral agents, interferon, retinoids, photodynamic therapy, zinc, COX2 inhibitors, vaccination, bevacizumab or gene therapy [Citation2,Citation13,Citation14]. Recently the use of systemic bevacizumab (Avastin®) has been suggested in case of aggressive and treatment-resistant RRP. Avastin® seems to reduce the growth of papilloma, prolong the inter-surgical interval, and improve the quality of life of patients with RRP [Citation15,Citation16]. Future studies are however necessary to determine dosing, treatment duration and response rate of Avastin® for RRP [Citation15,Citation16]. Therapeutic vaccination with the HPV vaccine also seems to prolong the intersurgical intervals for a significant proportion of RRP patients, but neither surgical nor medical treatment is curative and medical treatment is usually only adjuvant to surgery [Citation2,Citation13,Citation14]. However, in case of very widespread disease and/or pulmonary involvement, surgery may not be possible, leaving medical treatment as the only option.
The clinical course of RRP is highly variable and unpredictable, but as patients often require repeated treatment the disease is associated with reduced quality of life [Citation11].
Knowledge of the clinical course of RRP in the lower airways is limited. If the cystic bronchiectasis progresses it may cause reduced ventilation- and diffusion capacity, increased respiratory symptoms and frequent infections, and in worst case respiratory failure.
Conclusion
This case report illustrates that RRP is also a relevant differential diagnosis for respiratory physicians and should be considered in persons with the radiological presentation of bronchiectasis and especially in persons previously diagnosed RRP in the upper airways, who develop respiratory symptoms such as increasing dyspnea. It is also important not only to confirm the diagnosis of pulmonary RRP but to rule out other possible diagnoses. Patients with RRP should be identified as early as possible and monitored with regular intervals in order to detect early disease development and initiate relevant treatment of relapsing papillomas and possible malignant transformation.
Learning points
Recurrent respiratory papillomatosis (RPP) with pulmonary involvement is a very rare condition.
The HPV11 genotype is associated with more aggressive disease course compared to the HPV6 genotype.
There is currently no curative treatment for RRP.
Patients with RRP should be monitored regularly because of an increased risk of malignant transformation to squamous cell carcinoma.
Disclosure Statement
No potential conflict of interest was reported by the authors.
Additional information
Notes on contributors
Tatiana Mamaeva
Tatiana Mamaeva, MD, received her medical degree from the Volgograd Medical University, Russia in 2005. She is now a young doctor at the Department of Respiratory Medicine, Odense University Hospital, Denmark.
Camilla Slot Mehlum
Camilla Slot Mehlum, MD, PhD Fellow, completed her Medical degree from the University of Southern Denmark in 1998. She is a consultant at the Department of Otorhinolaryngology Head and Neck surgery, Odense University Hospital. Her main research areas are laryngology, voice and laryngeal precursor lesions.
Jesper Rømhild Davidsen
Jesper Rømhild Davidsen, MD, PhD, completed his Medical degree from the University of Southern Denmark in 2002, and his PhD in 2011. Dr. Davidsen is a consultant in internal and respiratory medicine, a Associate Professor at Department of Clinical Research, University of Southern Denmark, Denmark and leads the South Danish Center for Interstitial Lung Diseases at Department of Respiratory Medicine, Odense University Hospital, Denmark. His main research areas are within interstitial lung diseases, lung transplantation, and lung ultrasound.
References
- Gelinas JF, Manoukian J, Côté A. Lung involvement in juvenile onset recurrent respiratory papillomatosis: A systematic review of the literature. Int J Pediatr Otorhinolaryngol. 2008;72:433–5.
- Katsenos S, Becker HD. Recurrent respiratory papillomatosis: a rare chronic disease, difficult to treat, with potential to lung cancer transformation: apropos of two cases and a brief literature review. Case Report Oncol. 2011;4: 162–171.
- Mitsumoto GL, Barnardi F, Paes JF, et al. Juvenile-onset recurrent respiratory papillomatosis with pulmonary involvement and carcinomatous transformation. Autops Case Rep. 2018 Jul–Sep;8(3):e2018035.
- Kanazawa T, Fukushima N, Imayoshi S, et al. Rare case of malignant transformation of recurrent respiratory papillomatosis associated with human papillomavirus type 6 infection and p53 overexpression. Springerplus. 2013;2:153.
- Molodtsova V, Ryabova M, Dvorakovskaya I, et al. Recurrent respiratory papillomatosis with lung involvement. Respire Med Case Rep. 2018;25:323–326.
- Seedat RY, Shall R. Age of diagnosis, incidence and prevalence of recurrent respiratory papillomatosis-A South African perspective. Cain Otolaryngol. 2018 Apr;43(2):533–537.
- Zawadska-Glos L, Jakubowska A, Chmielik M, et al. Lower airway papillomatosis in children. Int J Pediatr Otorhinolaryngol. 2003 Oct;67(10):1117–1121. .
- Hyeon-Kyoung K, Vhul-Gui Y. Multiple cystic lung disease. Tuberc Respir Dis. 2013;74: 97–103.
- Francisco F, Souza AS Jr, Zanetti G, et al. Multiple cystic lung disease. Eur Respir Rev. 2015;24:552–564.
- Carifi M, Napolitano D, Morando M, et al. Recurrent respiratory papillomatosis: current and future perspectives. Dove Press. 2015;11:731–738.
- San Giorgi MRM, Aaltonen LM, Rihkanen H, et al. Quality of life of patients with recurrent respiratory papillomatosis. Laryngoscope. 2017;127:1826–1831.
- Ivancic R, Iqbal H, deSilva B, et al. Current and future management of recurrent respiratory papillomatosis. Laryngoscope Investig Otolaryngol. 2018;3:22–34.
- Gallagher TQ, Derkay CS. Pharmacotherapy of recurrent respiratory papillomatosis: an expert opinion. Expert Opin Pharmacother. 2009;10:645–655.
- Rosenberg T, Philipsen BB, Mehlum CS, et al. Therapeutic use of the HPV vaccine on recurrent respiratory papillomatosis: a systematic review and meta-analysis. J Infect Dis. 2019;219(7):1016–1025.
- Bedoya A, Glisinski K, Clarke J, et al. Systemic bevacizumab for recurrent respiratory papillomatosis: a single center experience of two cases. Am J Case Rep. 2017;18:842–846.
- Best SR, Mohr M, Zur KB. Systemic bevacizumab for recurrent respiratory papillomatosis: a national survey. Laryngoscope. 2017;127:2225–2229.
