?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Introduction
Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) has presented health-care systems worldwide with novel challenges and experiences and evidence is emerging during the pandemic. Patients requiring hospitalization frequently suffer from respiratory failure of different severities.
Aim
The aim of this guideline is the treatment of patients with SARS CoV-2 (COVID-19) in hospital; in particular, it addresses the treatment of respiratory failure treated in general Internal Medical- and Pulmonary Medical wards.
Results
Elderly patients and patients with chronic disease are particularly vulnerable to COVID-19. Target oxygen saturation should be between 92% and 96% in patients without chronic lung diseases. Treatment with >5 L oxygen/min should be in close collaboration with intensive care colleagues and >15 l/min preferably in intensive care units. High-flow nasal canula (HFNC) and long-term Continuous Positive Airway Pressure (CPAP) are recommended for patients not responding to conventional oxygen therapy. Non-invasive ventilation (NIV) is only recommended for selected patients, such as those with a ceiling of treatment or patients presenting with hypercapnic failure. With the use of humidification protective equipment as FFP2-3 masks should be used. Nebulized medication should be avoided, and spacers should be used instead.
Conclusion
Respiratory failure is frequently the cause of hospitalization in patients with COVID-19 and should be monitored closely.
About this guideline
This guideline is made by a consultancy under the Danish Society of Respiratory Medicine (DRSM) with the purpose of providing an overview over COVID-19 disease and its treatment during hospital admission in general and respiratory wards that is hospital wards with expertise in general Internal Medicine or specialized in Pulmonary Medicine but does not cover Intensive Care management of COVID-19. The guideline has a special focus on respiratory care, particularly oxygen treatment and respiratory support to guide hospital clinicians in respiratory care for the patient in a non-intensive care unit (ICU). For guidelines on intensive care therapy, please consult the Surviving Sepsis Campaign [Citation1].
In appendix 1 you will find a short pocket version, especially aimed at junior doctors, with a focus on the work in the emergency department.
Definition and prevalence
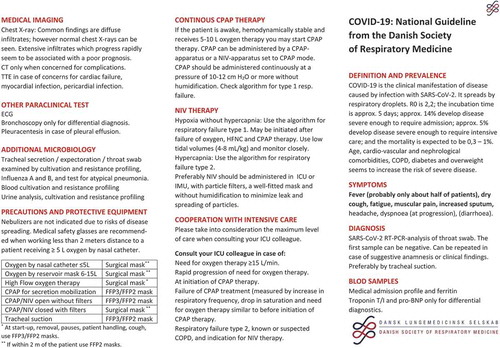
COVID-19 disease is caused by a pulmonary infection with the new coronavirus, severe acute respiratory infection coronavirus 2 (SARS-CoV-2). The virus was first identified during an outbreak of severe respiratory infections in December 2019 in the large Chinese city, Wuhan. The new coronavirus SARS-CoV-2 resembles SARS-CoV and MERS-CoV but has never previously been identified in humans. The virus is transmitted by droplets contact with contaminated surfaces. For SARS-CoV-2 the reproductive value, R0, is around 2.2 according to WHO (R0 is an indication of the transmissibility of a virus, representing the average number of new infections generated by an infectious person in a totally naïve population). The incubation period 2–14 days. Amongst those who contracted COVID-19, 15–20% require hospitalization, with around 15% of cases presenting with severe symptoms and 5% requiring intensive care [Citation2]. ICU mortality differs between studies but seems to be around 26% [Citation3]. The overall Case Fatality Rate (CFR) is around 4% but differs significantly between populations [Citation4].
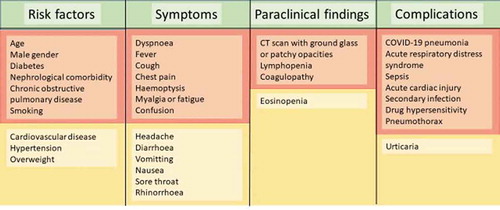
The most frequent symptoms are fever, dry or productive cough, fatigue and myalgia. Less frequent symptoms are cephalalgia, diarrhoea and, with disease progression, dyspnea and respiratory failure, which may even be tardive. Symptoms vary greatly amongst patients and may not be present at admission, e.g. fever is only seen in approximately half of the hospitalized patients. Symptoms are summarized in . Especially patients with chronic diseases as well as patients on immunosuppressive treatment regimens should be monitored closely, please consult paragraph 11.
Diagnostics
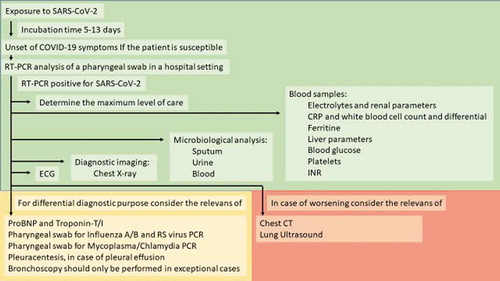
The diagnosis of COVID-19 with new coronavirus SARS-CoV-2 is obtained by a Reverse Transcription Polymerase Chain Reaction (RT-PCR) analysis of a pharyngeal swab. Hospitalized patients should be monitored, from arrival at the hospital with Early Warning Score (vitals including blood pressure, pulse rate, respiratory rate, oxygen saturation, temperature and responsiveness score) and a thorough physical examination must be performed. The following paraclinical tests are recommended:
Blood tests:
Electrolytes and renal parameters
CRP and white blood cell count and differential
Ferritin
Lactic Acid Dehydrogenase (LDH)
Liver parameters
Blood glucose
Platelets
Albumin
INR
Arterial blood gas analysis in patients with signs of respiratory insufficiency or dyspnea
Chest X-ray
ECG
Microbiological samples of sputum, urine and blood, as appropriate.
ProBNP and Troponin-T/I may be considered but should be requested in accordance with local recommendations.
Pharyngeal swab for coronavirus (note: First swab may be negative). If the patient is still suspected of COVID-19, isolation should be maintained, and PCR repeated after 24 h. As the pharyngeal swab may be a false negative, tracheal suctioning may be considered if the patient has a productive cough.
Consider
Pharyngeal swab for Influenza A/B and RS virus PCR
Pharyngeal swab for Mycoplasma/Chlamydia PCR
It is recommended that diagnostic samples are obtained at initial contact with the patient and before the patient is moved to the general ward. Depending on the condition of the patient and diagnostic results other investigative measures may be considered:
Bronchoscopy should only be performed in exceptional cases and only for differential diagnostic purposes.
Pleuracentesis, in case of pleural effusion, should be carried out bedside to avoid the risk of contamination.
The diagnostic work-up is summarized in .
Radiological modalities
Chest X-ray should be performed in all patients admitted with suspicion of COVID-19. Chest CT is rarely indicated in the initial phase of the disease unless other pathology, requiring CT, is suspected. Focused lung ultrasound and general lung ultrasound (F-LUS/LUS) is useful to monitor disease progression and to diagnose complications at the bedside with minimal exposure of disease to fellow patients and health-care personnel.
Chest X-ray
Chest X-ray may be normal despite symptoms requiring hospitalization. Typical radiological changes are diffuse infiltrates. Rapidly progressive radiological changes are associated with poor prognosis and the extent of the radiological changes also is negatively associated with prognosis [Citation5].
CT scan
Even patients with minimal symptoms may display multiple radiological changes on chest CT. In a study from Wuhan, China, it was demonstrated that pathological findings could be found in all pulmonary lobes, with a tendency towards the right lower lobe being more frequently involved. Bilateral changes were seen in 79% of patients, 54% had peripheral ground glass changes and 44% had diffuse changes. The most typical findings were ground glass changes (65%), interlobular septal thickening (35%), air-bronchograms (47%), crazy paving (10%) and pleural thickening. Pleural effusion was rare and only seen in 5% of patients [Citation6].
Lung ultrasound (F-LUS/LUS)
Lung ultrasound is very useful in patients with COVID-19. It may be used for radiological follow-up and to acknowledge complications as consolidation, atelectasis, pleural effusion, and pneumothorax [Citation7].
A small Chinese study has investigated ultrasound findings in patients with COVID-19 [Citation8]. The most frequent findings were 1) thickening of the pleura, 2) B-lines in various patterns, amongst those; focal, multiple and confluent 3) consolidations and air-bronchograms and 4) regression of B-lines with recovery and in continuation with this, dominant A-line patterns.
Considerations about the extent of care
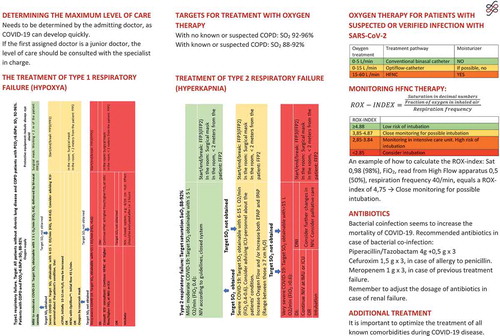
In all patients, and especially in those suspected of COVID-19, the extent of care should be considered at admission. A decision should be made by the admitting doctor, and in case this is a junior doctor, a senior doctor should be involved in the decision.
The choice of the ceiling of treatment has a great impact on the choice of strategy for oxygenation and respiratory support. In general, in patients where health conditions allow intubation (Do Intubate (DI) patients), with hypoxic respiratory failure (type 1 respiratory failure), who do not respond to oxygen therapy at moderate flow (up to 15 l/min) referral to the Intensive Care Unit (ICU) should be considered. The ICU doctor should be advised about patients with clinical signs of disease progression and progressive respiratory failure, requiring ≥5 L of oxygen/minute. If the patient has been considered a candidate for intubation, patients with hypercapnic respiratory failure (type 2 respiratory failure) and COPD should be intubated if the patient does not respond sufficiently to non-invasive ventilation (NIV).
Oxygen treatment and respiratory support
All units treating COVID-19 patients may be involved in treatment with oxygen and respiratory support. A compromised diffusion capacity and development of ventilation-perfusion-mismatches, as a result of progressively affected lower airways, may lead to respiratory insufficiency and respiratory failure. In general, four types of respiratory failure are described:
Type 1 respiratory failure (hypoxic failure)
Type 2 respiratory failure (hypercapnic failure)
Type 3 respiratory failure (postoperatively)
Type 4 respiratory failure (following hypo-perfusion and circulatory failure)
The most frequent types of respiratory failure seen in patients with COVID-19 in general wards will be type 1 and, to a lesser extent, type 2 or a combination of those, and will, therefore, be discussed in detail below.
Hypoxic respiratory failure (type 1)
Oxygen/O2
To date, no evidence of optimal oxygen treatment for COVID-19 patients is known, neither in terms of a method for administration nor for target saturation (SpO2).
Oxygen should be administered through a conventional bi-nasal cannula or a high-flow nasal cannula in patients with a need for a fraction of inspired oxygen (FiO2) <40%. Humidification should be avoided to minimize environmental aerosols and thereby minimize the risk of spreading the disease. With a need of FiO2 > 40% (equivalent to approx. 5 L/min on ordinary bi-nasal cannula), flow can be increased, still without humidification, to 6 to 15 L/min by using a high-flow nasal cannula, thus achieving a FiO2 of approximately 40 to 60%. For achieving higher FiO2, please consult the paragraph below on high-flow nasal cannula (HFNC).
WHO recommends that in adult, non-pregnant patients with COVID-19, target SpO2 should be >90% when the patient is stabilized, while in critically ill patients (with shock, coma, seizures, risk of respiratory arrest), an SpO2 > 94% should be the target [Citation9]. British guidelines for acute oxygen treatment recommend that in acute critically ill patients without risk of hypercapnia target SpO2 should be 94–98% [Citation10]. A meta-analysis from Lancet, including 16.037 patients, demonstrated increased mortality in patients treated with liberal oxygen treatment compared to conservative oxygen treatment, though most studies were based on patients with acute cerebral- or coronary ischemia [Citation11]. Consistent results were seen in a smaller study in 480 ICU patients [Citation12].
The Danish Health Authorities published National recommendations for oxygen treatment of adult patients in an acute setting, with a weak recommendation of oxygen treatment targeted at SpO2 ≥ 94% and a weak recommendation for titration of oxygen to a target SpO2 between 94 and 98% for patients with acute illness and an SpO2 < 94% [Citation13]. A study in patients with acute respiratory distress syndrome (ARDS), randomized to intensive care and SpO2 88–92% versus SpO2 > 96%, indicated an increased mortality at the lower SpO2 target, and the study was terminated before enrolment was completed [Citation14]. The Surviving Sepsis Campaign guidelines on COVID-19 does not recommend SpO2 < 90%, nor above 96%, and recommend target SpO2 between 92 and 96%. Patients with COPD and COVID-19 should be treated with oxygen, according to usual recommendations, with target SpO2 between 88 and 92% [Citation15].
DSRM recommends:
-That target SpO2 in COVID-19 patients without known chronic lung disease should be 92–96%
-That target SpO2 in COVID-19 patients with known chronic lung disease (COPD) should be 88–92%
In patients with type 1 respiratory failure requiring FiO2 > 40% an increase of oxygen flow to 6–15 L/min is recommended. Flow in this range can be delivered without humidification and by nasal cannula used for high flow. Consider therapy with either continuous CPAP or HFNC or oxygen via a mask with a reservoir (). These treatment modalities are considered equal.
Table 1. Flowchart for the treatment of type 1 respiratory failure and choice of protectiveequipment.
If possible, oxygen humidification should be avoided to reduce particle contamination in the environment. Recommendations are shown in .
Table 2. Recommendations on humidification of oxygen delivery for patients suspected of- or diagnosed with COVID-19.
High-flow nasal cannula (HFNC)
HFNC treatment may, according to the WHO recommendations, be considered in type 1 respiratory failure in COVID-19 patients [Citation9]. As such, HFNC may be used both in DI-patients and patients not recommended for intubation (Do Not Intubate (DNI)).
HFNC has previously been shown useful for milder cases of ARDS, with less than one out of five patients in need for treatment escalation to intubation [Citation16]. During the COVID-19 pandemic, there have been concerns about environmental particle distribution from HFNC, but in a recent study particle distribution to the environment has been shown to be less than for oxygen treatment with 5 L/min on ordinary bi-nasal cannula, even at an HFNC-flow of 60 L [Citation17,Citation18]. DRSM recommends fitting the largest possible Optiflow™- cannula in the individual patient.
For monitoring patients treated with HFNC the Respiratory Rate oxygenation (ROX)-index can be used, calculated as [Citation16]. A ROX index >4.88 indicates a low risk of intubation, and thereby use of HFNC can continue, whereas a ROX-index <2.85 should lead to intubation if DI has been decided. At values in between patients should be monitored closely [Citation19]. The index was used during the COVID-19 epidemic in Wuhan for monitoring HFNC-treated patients () [Citation20].
Figure 3. ROX-Score for evaluation of oxygen treatment of type 1 respiratory failure, treated with High-Flow treatment.
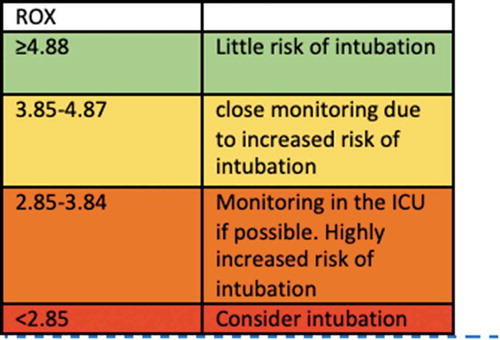
An example of a ROX-index calculation: SpO2: 90%, FiO2: 0.70, RR: 30 ((90/0.7)/30) = ROX-index of 4.28.
FiO2 can be read from the display of the high-flow device.
Ordinary-heated humidifiers may be used as an alternative, should HFNC not be available, preferably with an Optiflow™ nasal cannula. Please note that this will generate considerably more aerosol distribution to the surroundings and should be accompanied using suitable protective equipment, i.e. FP2/FFP3 masks.
CPAP
Continuous Positive Airway Pressure (CPAP) may be administered through different equipment at variable pressures and duration. In principle, we distinguish between intermittent CPAP (iCPAP) and continuous CPAP (cCPAP). iCPAP may be administered using single-patient use equipment or simple electric devices. cCPAP should be administered through dedicated CPAP-equipment or NIV-ventilators in CPAP-mode.
Evidence of the effect of iCPAP is modest as studies are small and the methodology used is weak [Citation21]. iCPAP may be used for the treatment of atelectasis and mucus clearance; probably by improving collateral ventilation [Citation21]. iCPAP increases Functional Residual capacity (FRC) and recruit alveoli; however, the effect only lasts while iCPAP is applied to the patient and will therefore only be modest if iCPAP only is used for minutes, at hours’ interval.
cCPAP has been used as a home treatment of obstructive sleep apnoea (OSA) and for manifest heart failure, where it reduces mortality and need for intubation equivalent to NIV. cCPAP increases FRC and recruit alveoli; reduces dead space ventilation and re-expand liquid-filled alveoli [Citation22].
The WHO guideline for COVID-19 does not recommend CPAP for adult patients with COVID-19 [Citation9]. Surviving Sepsis Campaign recommends treatment with high PEEP to patients with COVID-19 and ARDS, which has proven superior to treatment with low PEEP during mechanical ventilation of ARDS due to other conditions [Citation1]. As PEEP and CPAP physiologically have approximately the same effect; CPAP at relatively high pressures (>10 cm H2O) should be considered [Citation21].
The Italian guidelines for COVID-19 by the Italian Thoracic Society and Italian Respiratory Society recommend cCPAP at 10–12 cm H20, which may be increased to 15–20 cm H2O in patients not responding sufficiently to concomitant oxygen flows at 10–15 L [Citation23]. cCPAP is often necessary continuously for several days in patients with COVID-19. CPAP is preferably administered by ”helmet” to reduce the environmental spread of aerosols; however, experience with this kind of treatment in Denmark is sparse, and thus CPAP by mask must be applied with appropriate precautions for contamination () [Citation23].
Table 3. Flowchart for treatment and choice of protective equipment in type 2 respiratoryfailure.
In general, iCPAP cannot be expected to have any major clinical effect on COVID-19 or ARDS and should only be used in the context of its usual indication (atelectasis, mucus clearance). cCPAP has physiologically beneficial effects on gas exchange in patients with COVID-19 and may be used in patients not responding to oxygen therapy. Pressures of 10–12 cm H2O or higher should be used. Please note that use of cCPAP in a general ward requires close monitoring of the patients, who should be awake and circulatory stable. The effect of cCPAP should be noticeable immediately with a decrease in respiratory rate and decrease in required oxygen therapy; otherwise, ICU should be consulted. If oxygen requirements should increase again after they initially reduced, intubation should also be considered. If the ward staff is inexperienced in the use of cCPAP, treatment should take place in ICU or equivalent experienced department.
BiPAP/NIV
Use of Bilevel Positive Airway Pressure/NIV for the treatment of hypoxic failure in patients with COVID-19 is poorly described, there are no randomized trials to date. Previous studies have focused on the use of NIV for viral pneumonias and hypoxic respiratory failure with reports on treatment failure rates of 30–33% and in a more recent study on H1N1 influenza virus infections, with treatment failure rates between 13 and 77% [Citation24].
In general, the use of NIV for hypoxic failure should be carried out in intermediate care units (IMU) or ICU where intubation and mechanical ventilation is at hand. NIV with an open circuit system is highly contaminating the surrounding environment in terms of potential infective particles. Therefore, an anti-virus filter should be used on the exhalation port, placed on the tube and not on the mask (). In DNI-patients NIV should be considered in case of the inadequate effect of oxygen, HFNC, and CPAP, but should be administered under close monitoring and only at low tidal volumes (4–8 mL/kg) [Citation1].
Figure 4. Closed system CPAP/NIV: Non-vented mask – filter – exhalation port – tube-filter ventilator.

Due to increased risk of particle contamination of the environment, NIV should be used without humidification in patients with COVID-19. Furthermore, awareness of mask fitting is recommended to diminish particle distribution to the environment.
In patients with COVID-19 using domiciliary NIV humidification should not be used if home care is provided to the patient.
In all other cases, NIV should be used according to national guidelines. For type 2 respiratory failure, please consult .
Hypercapnic respiratory failure (type 2)
Patients with COPD-developing exacerbation (AECOPD) are at risk of developing hypercapnic respiratory failure and respiratory acidosis. This should be treated with NIV according to GOLD recommendations [Citation15,Citation25]. Patients with asthma and without diagnosed chronic lung disease may develop type 2 respiratory failure. This should be treated in ICU.
Nebulised medication
Particle contamination of the environment is considerable during the use of nebulized medication [Citation26,Citation27], especially when administered through a mask [Citation18]. However, also administration through a mouthpiece causes substantial contamination, which may be reduced using a particle filter [Citation28].
Disease transmission through the use of nebulized medication has been shown, both during outbreaks of Influenza and SARS [Citation29–Citation31] and has also been suggested in COVID-19 [Citation32]. Therefore, the use of nebulized medication, unless when administered through a particle filter, is not recommended during the COVID-19 epidemic [Citation33].
The following systems may be used for administration of short-acting beta-2-agonist (SABA) and/or short-acting muscarinic antagonists (SAMA) in patients who are incapable of using usual inhalers:
Use of spacer with mask
Administration of inhaled SABA/SAMA is equal to nebulization through mouthpiece, superior to nebulization through mash yet inferior to nebulization using MESH-technology [Citation34,Citation35].
Use of spacer treatment requires minimal cooperation from the patient. Consider the use of a spacer with the mouthpiece in patients who can cooperate.
COVID-19 complications
A number of complications to SARS-CoV-2 disease have been recognized so far [Citation6,Citation36,Citation37], and different patient categories have different susceptibilities. An overview is given in , and certain aspects, such as bacterial complications and patients with chronic diseases will be addressed in detail below.
Antimicrobial treatment
Antiviral treatments against Coronavirus
No specific antiviral agents have been developed against COVID-19. Recently, in a randomized trial, the combination Lopinavir–Ritonavir (protease inhibitor + agent to increase bioavailability) has been investigated in 199 adult patients with severe COVID-19 respiratory infection, compared to standard care. After 28 days, there was no difference in mortality rate between groups. Treatment is not recommended in April 2020 [Citation38]. Treatment with Hydroxychloroquine as immune regulatory- and antiviral agent has been suggested in COVID-19. Hydroxychloroquine has been known since the 1930ʹies and is used as an anti-inflammatory treatment of rheumatic diseases and for prophylaxis and treatment of malaria, but also as an anti-viral treatment of, e.g. Flavivirus, Retrovirus, and Coronavirus, by an inhibitory effect on virus replication [Citation39]. Hydrochloroquine has been well tolerated even in long-term use and no teratogen effect has been described. In vitro cell studies in primate cells infected with the coronavirus SARS-1 indicated a dose-respondent, inhibitory effect of hydrochloroquine [Citation40]. There are ongoing studies investigating the effect of hydroxychloroquine/chloroquine in COVID-19 disease [Citation41,Citation42]. Off label use of hydrochloroquine is therefore not recommended.
Anti-bacterial agents
Antibacterial agents are of no use against viral infections; however, they are useful in the treatment of suspected/verified secondary bacterial infections. A previous study showed that 50% of patients who died of COVID-19 had secondary infection, whereas only 1% of patients who survived had had a secondary infection (p < 0.0001) and septic shock was seen in 70%/0% in the respective groups [Citation37]. There are no solid data on which microorganisms are most prevalent in secondary infections. However, it is reasonable to believe that bacterial infections are most prevalent, and variable within the patient population, co-dependent on both pulmonary (e.g. bronchiectasis and COPD) and other chronic comorbidities (e.g. diabetes, treatment with immunosuppressants). As such, there is reason to believe that secondary bacterial infections are important for disease development and the patient’s prognosis.
It is therefore recommended that at any given time, at admission or during hospitalization, if a secondary bacterial infection is suspected, antibiotic treatment should be initiated. Treatment should be broad-spectrum, as the most common aetiology of secondary infections in COVID-19 is yet unknown, and since secondary infections are often caused by bacteria resistant to small-spectrum antibiotics. The diagnostic work-up does not differ from that in other patients; suspected bacterial infection and relevant material for microbiological investigation should be secured before the start of antibiotic treatment. The most frequent infection is probably pneumonia and it is unknown whether the secondary bacterial pneumonias should be considered equivalent to community-acquired pneumonia or hospital-acquired pneumonia. Furthermore, disease progression in case of secondary bacterial infection has been rapid. The empiric recommendation is therefore:
Pneumonia (suspected or confirmed)
Non-ICU with oxygen need ≤5 LO2/min and/or FiO2 ≤ 0.4:
Tolerant to beta-lactam antibiotics:
Inj. Piperacillin/Tazobactam 4 g + 0.5 g x 4 i.v. *
Intolerant to beta-lactam antibiotics:
Inj. Cefuroxim 1.5 g x 3 i.v.
Treatment failure (2–3 days with no significant clinical or para-clinical improvement):
Inj. Meropenem 1 g x 3 i.v. *
* Dose dependence on renal function and body weight, and should be corrected accordingly.
ICU-patients or oxygen need ≥5 LO2/min and/or FiO2 ≥ 0.4
Please consult the local recommendations for antibiotic treatment in an ICU-setting
Treatment of Sepsis
Please consult the Surviving Sepsis campaign COVID-19 guidelines [Citation1]. Furthermore, a recent publication includes a flow chart for handling critically ill patients with COVID-19 [Citation43].
Concerning septicemia and COVID-19, a publication on the Coronavirus MERS showed that 20–25% of critically ill patients had bacterial co-infection [Citation44].
In patients with parallel infections with other potent viral agents, such as influenza, pulmonary secondary bacterial infections with S. aureus have been shown to be very prevalent and therefore this micro-organism should be covered by the antibiotics of choice [Citation45].
The most prevalent microorganisms cultured from the lungs of septic patients in the ICU as secondary agents have been K. pneumoniae, S. marscecens and P. aeruginosa.
Other treatments – ARDS
A described complication to COVID-19 is Acute Respiratory Distress Syndrome (ARDS). ARDS is most frequently diagnosed by the so-called Berlin criteria [Citation46]:
Bilateral ground-glass changes/opacities/consolidations
No other identified cause of these changes
Severe type 1 respiratory Failure
>7 days since primary insult
The condition is very difficult to treat, and patients are treated in the ICU. A recent study indicated a possible beneficial effect of dexamethasone [Citation47]. However, this is not recommended. A single RCT showed that prone positioning of the patient has been shown to reduce 28 days-mortality from 33% to 16% (p < 0.0001) [Citation48]. This modality has been used in the treatment of patients with COVID-19 [Citation49], however, results specific to COVID-19-related ARDS are yet unknown.
Procalcitonin (PCT)
There are very few reports on initial PCT levels at admission in patients with COVID-19 [Citation50,Citation51]. A low PCT (<0.5 ng/ml) is seen in 95% of patients and median PCT is low 0.13 ng/ml, independent of oxygen saturation. There are no systematic studies of sequential PCT measurements in patients with COVID-19, and therefore it is unclear whether PCT would be of use in early detection of secondary bacterial infections. Previously, in vitro studies have shown that IFNγ is inhibitory of the PCT response [Citation52], which gives us reason to hypothesize that infection with SARS-CoV-2 is inhibitory of a PCT response, even in case of secondary bacterial infection. Therefore, as of now, we do not recommend neither initial nor sequential use of PCT measurements in hospitalized COVID-19 patients.
COVID-19 and comorbidities
Critical COVID-19 illness has been shown to be significantly more prevalent in elderly and immunosuppressed patients, as well as those with comorbidities [Citation37]. In a recent, small study from Washington [Citation53] 85% of patients had one or more comorbidities, which is in line with previous studies [Citation9,Citation54,Citation55]. Most prevalent were renal failure (48%), heart failure (42%), COPD and diabetes (33%), and OSA (28%). It is also noticeable that time-to-intubation among hospitalized patients was only 1.5 days. It may both reflect pre-hospital routines, but also reflect a significant vulnerability to the disease in patients with the above-mentioned chronic diseases. It has been hypothesized that a cause of the vulnerability was treatment with ACE-inhibitors [Citation56] as one of the virus-receptors’ is ACE2 [Citation57]; however, patients are, for now, not advised to pause treatment. A retrospective study of fatal cases in a population from Wuhan showed that 23% of patients had renal failure and 29% had heart failure [Citation58]. It remains unclear whether patients with asthma and allergies are at special risk, but Global Initiative for Asthma recommends patients to be well treated at all times [Citation33]
Apart from respiratory comorbidities, special attention should, therefore, be paid to patients with:
Cardiovascular comorbidities
Renal comorbidities
Diabetes (and obesity)
In patients with cardiovascular disease, special attention should be on progression in ischemic symptoms and myocarditis, and in renal disease progression of renal failure and acute renal failure. A thorough glucaemic control is recommended in diabetes and adjustment in ventilatory requirements to meet the need of the obese patients is essential.
Patients with respiratory comorbidities should continue the usual treatment of their chronic respiratory diseases.
Isolation
Transmission of SARS-CoV-2 via aerosols and direct contact is possible as the virus is viable in aerosols for hours and on surfaces for days [Citation59]. As such, there is a risk of nosocomial disease transmission to other patients and to health-care personnel. Therefore, the patient suspected of COVID-19 should be isolated from the moment of arrival at the Hospital.
A suitable distance between patients with suspected- or confirmed SARS-CoV-2 is at least 1 meter [Citation9]. In patients receiving different types of oxygen therapy, protective equipment should be taken within 2 meters of the patient. It is recommended to use surgical masks for symptomatic patients during the examination and during transportation, although the transportation of patients should be minimized. Patients suspected of infection with SARS-CoV-2 should be placed in a single isolation room. When the diagnosis is confirmed, cohort isolation is possible. Isolation can be terminated when the patient has been asymptomatic for 48 h. However, it has been suggested that a patient can transmit virus more than 48 h after termination of symptoms and contact to vulnerable subjects for a longer period should be avoided until the matter has been clarified.
Hospital staff should be thoroughly instructed to prevent infections, including the use of personal protective equipment [Citation9,Citation43]. The number of staff and the number of procedures carried out in a ward with SARS-CoV-2-infected patients should be limited; however, patient’s safety and well-being should not be compromised.
Protective equipment
Standard precautions should always be used in all health facilities whenever caring for a patient suspected of- or diagnosed with COVID-19. These include hand hygiene and use of personal protective equipment when in direct- and indirect contact with patient’s blood, body fluids, and secretions, as well as non-intact skin [Citation9].
Use of single use or dedicated equipment is preferred (i.e. stethoscopes, thermometer,s etc.). If the equipment is shared between patients, it should be cleaned and disinfected between use.
Protective equipment (mask, protective glasses, gloves and long-sleeved, water-repellent single-use coat) should be worn when entering the patient’s room. Protective equipment should be removed before leaving the patient’s room and thorough hand hygiene should be carried out.
FFP3 or FFP2 masks are used during aerosol-producing procedures, i.e. tracheal suction, intubation, CPAP, NIV, HFNC, and bronchoscopy [Citation60,Citation61]. FFP2 masks and protective glasses are recommended when working within 2 m of patients receiving ≤5 L oxygen on bi-nasal cannula. Special care should be taken within 2 m of the patients using HFNC or CPAP/NIV due to the risk of environmental contamination with aerosols. NIV with single tube systems may be used applying an anti-viral filter to the exhalation port (). In addition, a filter is applied between the device and the tube. The mask must be non-ventilated, and well fitted to avoid air leak and aerosol spread. A number of studies have demonstrated no disease transmission to health-care personnel from patients treated with NIV if these appropriate precautions were taken into account [Citation62,Citation63].
Recommendations for protective equipment in connection with different oxygen treatment modalities are resumed in .
Table 4. Recommendations for the use of protective equipment when caring for patients with oxygen need and need of airway handling.
Palliative care
Recommendations for palliative care for patients with COVID-19 does not differ from recommendations in general, we, therefore, recommend the use of local guidelines or consult [Citation64]. However, special care should be taken of patients’ relatives who due to the risk of spreading disease do not have the usual possibilities for being with their loved ones.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Notes on contributors
Klaus Nielsen Jeschke
Klaus Nielsen Jeschke is a Senior Consultant at the Department of Respiratory Medicine at Hvidovre Hospital. He specialises in NIV and is also the first author of the Danish COVID guideline.
Barbara Bonnesen
Barbara Bonnesen is a Senior House Officer. She has special interest in infections in patients with obstructive lung diseases.
Ejvind Frausing Hansen
Ejvind Frausing Hansen is a Senior Consultant at the Department of Respiratory Medicine at Hvidovre Hospital. He specialises in treatment of Respiratory Failure, which is also his field of research.
Jens-Ulrik Stæhr Jensen
Jens-Ulrik Stæhr Jensen is Senior Consultant and Head of Research at the Department of Respiratory Medicine at Hvidovre Hospital. He is the founder of the Danish COP-TRIN network, performing research in COPD exacerbation. Furthermore his field of research has been pulmonary infections.
Therese Sophie Lapperre
Therese Sophie Lapperre is a Senior Consultant at the Department of Respiratory Medicine at Bispebjerg Hospital. She specialises in treatment of COPD. Her field of research is the effect of corticosteroids on airway inflammation in COPD.
Ulla Møller Weinreich
Ulla Møller Weinreich is a Senior Consultant and Head of Research at the Department of Respiratory Diseases at Aalborg University Hopsital. She is a member of the board of the COP-TRIN network and the Danish Trial Nation Network and vice chairman of the DSRM. Her field of research is High Flow treatment and COPD and comorbidities.
Ole Hilberg
Ole Hilberg is Professor of Pulmonary Medicine at the Department of Respiratory Medicine at Vejle Hospital. He has a solid background in pulmonary research in interstitial lung diseases, COPD, Asthma and is the founder of the Danish Center for Lung Cancer Research. OH is chairman of DSRM.
References
- Oczkowski S, Levy MM, Derde L, et al. Surviving sepsis campaign : guidelines on the management of critically Ill adults with coronavirus disease 2019 (COVID-19). Authors Intensive Care Medicine (ICM) Crit Care Med. 2020;2019;1–3.
- World Health Organization (WHO). Health systems respond to COVID-19 technical guidance #2 creating surge capacity for acute and intensive care recommendations for the WHO European Region (6 April 2020), The World Health Organisation; 2020.
- Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 PATIENTS INFECTED WITH SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. Jama. 2020;1–15. DOI:10.1001/jama.2020.5394.
- Spychalski P, Błażyńska-Spychalska A, Kobiela J. Correspondence Estimating case fatality rates of COVID-19. Lancet. 2020;3099:19–20.
- Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513.
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506.
- Laursen CB, Rahman NM, Volpicelli G, editor. Thoracic Ultrasound. Eur Respir Soc. 2018. DOI:10.1183/2312508X.erm7918
- Peng QY, Wang XT, Zhang LN, et al. Findings of lung ultrasonography of novel corona virus pneumonia during the 2019 – 2020 epidemic. Intensive Care Med. 2020;6–7. DOI:10.1007/s00134-020-05996-6
- World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected. Vol. 2019, The World Health Organisation; 2020. p. 12.
- O’Driscoll BR, Howard LS, Earis J, et al. BTS guideline for oxygen use in adults in healthcare and emergency settings. Thorax. 2017;72:ii1–90.
- Chu DK, Kim LHY, Young PJ, et al. Mortality and morbidity in acutely ill adults treated with liberal versus conservative oxygen therapy (IOTA): a systematic review and meta-analysis. Lancet. 2018;391:1693–1705.
- Girardis M, Busani S, Damiani E, et al. Effect of conservative vs conventional oxygen therapy on mortality among patients in an intensive care unit the oxygen-ICU randomized clinical trial. J Am Med Assoc. 2016;316:1583–1589.
- Sundhedsstyrelsen. National Klinisk Retningslinje for iltbehandling til den akut syge voksne patient. 2019.
- Barrot L, Asfar P, Mauny F, et al. Liberal or conservative oxygen therapy for acute respiratory distress syndrome. N Engl J Med. 2020;382:999.
- López-Campos JL, Soler-Cataluña JJ, Miravitlles M. global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2019 report: future challenges. Arch Bronconeumol. 2020;56:65–67.
- Messika J, Ben AK, Gaudry S, et al. Use of high-flow nasal cannula oxygen therapy in subjects with ARDS: a 1-year observational study. Respir Care. 2015;60:162–169.
- Hui DS, Chow BK, Lo T, et al. Exhaled air dispersion during high-flow nasal cannula therapy versus CPAP via different masks. Eur Respir J. 2019;53. DOI:10.1183/13993003.02339-2018.
- Hui DSC, Chan MTV, Chow B. Aerosol dispersion during various respiratory therapies: a risk assessment model of nosocomial infection to health care workers. Hong Kong Med J. 2014;20:9–13.
- Roca O, Caralt B, Messika J, et al. An index combining respiratory rate and oxygenation to predict outcome of nasal high-flow therapy. Am J Respir Crit Care Med. 2019;199:1368–1376.
- Ruiqiang Z, Ming H, Care I. Norms and advice expert advice on procedure of respiratory therapy for severe novel coronavirus pneumonia. Chin J Crit Care Intensive Care Med. 2020. DOI:10.3877/cma.j..2096-1537.2020.E001
- Denehy L, Berney S. The use of positive pressure devices by physiotherapists. Eur Respir J. 2001;17:821–829.
- Xu XP, Zhang XC, Hu SL, et al. Noninvasive ventilation in acute hypoxemic nonhypercapnic respiratory failure: a systematic review and meta-analysis. Crit Care Med. 2017;45:e727–33.
- Italian Thoracic Society and Italian Respiratory Society. Managing the Respiratory care of patients with COVID-19; 2020.p. 1–17.
- Rochwerg B, Brochard L, Elliott MW, et al. Official ERS/ATS clinical practice guidelines: noninvasive ventilation for acute respiratory failure. Eur Respir J. 2017;50. DOI:10.1183/13993003.02426-2016.
- Hansen EF, Fabricius P, Titlestad IL, et al. KOL - exacerbation og NIV. Lungemedicinsk Selsk 2017:1–5.
- Simonds AK, Hanak A, Chatwin M, et al. Evaluation of droplet dispersion during non-invasive ventilation, oxygen therapy, nebuliser treatment and chest physiotherapy in clinical practice: implications for management of pandemic influenza and other airborne infections. Health Technol Assess (Rockv). 2010;14:131–172.
- Davies A, Thomson G, Walker J, et al. A review of the risks and disease transmission associated with aerosol generating medical procedures. J Infect Prev. 2009;10:122–126.
- McGrath JA, O’Sullivan A, Bennett G, et al. Investigation of the quantity of exhaled aerosols released into the environment during nebulisation. Pharmaceutics. 2019;11:1–9.
- Gamage B, Moore D, Copes R, et al. Protecting health care workers from SARS and other respiratory pathogens: a review of the infection control literature. Am J Infect Control. 2005;33:88–96.
- Hui DS, Chow BK, Chu LCY, et al. Exhaled air and aerosolized droplet dispersion during application of a jet nebulizer. Chest. 2009;135:648–654.
- Ibrahim E, Harnish D, Kinney K, et al. An experimental investigation of the performance of a Collison nebulizer generating H1N1 influenza aerosols. Biotechnol Biotechnol Equip. 2015;29:1142–1148.
- Amirav I, Newhouse MT. Transmission of coronavirus by nebulizer: a serious, underappreciated risk. Can Med Assoc J. 2020;192:E346–E346.
- GINA. No Title. FAQ Asthma COVID-19 2020. [cited 2020 Apr 14]. Available from: https://ginasthma.org/covid-19-gina-answers-to-frequently-asked-questions-on-asthma-management/
- Respaud R, Vecellio L, Diot P, et al. Nebulization as a delivery method for mAbs in respiratory diseases. Expert Opin Drug Deliv. 2015;12:1027–1039.
- Pritchard JN, Hatley RHM, Denyer J, et al. Mesh nebulizers have become the first choice for new nebulized pharmaceutical drug developments. Ther Deliv. 2018;9:121–136.
- Zhang J, Dong X, Cao Y, et al. Clinical characteristics of 140 patients infected with SARS‐CoV‐2 in Wuhan, China. Allergy. 2020;all.14238. DOI:10.1111/all.14238.
- Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062.
- Cao B, Wang Y, Wen D, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;1–13. DOI:10.1056/NEJMoa2001282.
- Savarino A, Boelaert JR, Cassone A, et al. Effects of chloroquine on viral infections: an old drug against today’s diseases? Lancet Infect Dis. 2003;3:722–727.
- Vincent MJ, Bergeron E, Benjannet S, et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2:1–10.
- Colson P, Rolain JM, Lagier JC, et al. Chloroquine and hydroxychloroquine as available weapons to fight COVID-19. Int J Antimicrob Agents. 2020;55:105932.
- Gao J, Tian Z, Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 2020;14:72–73.
- Murthy S, Gomersall CD, Fowler RA. Care for Critically Ill Patients With COVID-19. Jama. 2020;18–19. DOI:10.1001/jama.2020.3633
- Alhazzani W, Møller MH, Arabi YM. et al. surviving Sepsis Campaign: guideline on critically ill adults with Coronavirus Disease 2019 (COVID-19) Intensive Care Med. 2020,1–34.
- Uyeki TM, Bernstein HH, Bradley JS, et al. Clinical practice guidelines by the Infectious Diseases Society of America: 2018 update on diagnosis, treatment, chemoprophylaxis, and Institutional Outbreak Management of Seasonal Influenza. Clin Infect Dis. 2019;68:895–902.
- Ferguson ND, Fan E, Camporota L, et al. The Berlin definition of ARDS: an expanded rationale, justification, and supplementary material. Intensive Care Med. 2012;38:1731–1732.
- Villar J, Ferrando C, Martínez D, et al. Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir Med. 2020;8:267–276.
- Guérin C, Reignier J, Richard JC, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368:2159–2168.
- Meng L, Qiu H, Wan L, et al. Intubation and ventilation amid the COVID-19 outbreak: Wuhan’s experience. Anesthesiology. 2020. DOI:10.1097/ALN.0000000000003296.
- Guan W-J, Ni Z-Y, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;1–13. DOI:10.1056/NEJMoa2002032.
- Zhao D, Yao F, Wang L, et al. A comparative study on the clinical features of COVID-19 pneumonia to other pneumonias. Clin Infect Dis. 2020;ciaa247. DOI:10.1093/cid/ciaa247.
- Linscheid P, Seboek D, Nylen ES, et al. in vitro and in vivo calcitonin I gene expression in parenchymal cells: a novel product of human adipose tissue. Endocrinology. 2003;144:5578–5584.
- Arentz M, Yim E, Klaff L, et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. Jama. 2020;4720:2019–2021.
- Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. Jama. 2020;2019:3–6.
- Young BE, Ong SWX, Kalimuddin S, et al. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. Jama. 2020;1–7. DOI:10.1001/jama.2020.3204.
- Bulletin ACCC. COVID-19 Clinical Guidance For the Cardiovascular Care Team; n.d..p. 1–4.
- Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor article SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:1–10.
- Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;2600:1–7.
- van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and surface stability of SARS-CoV-2 as COMPARED with SARS-CoV-1. N Engl J Med. 2020 Mar 17. [Epub Ahead Print] PubMed PMID 32182409 n.d. DOI:10.1056/NEJMc2004973
- Tellier R, Li Y, Cowling BJ, et al. Recognition of aerosol transmission of infectious agents: a commentary. BMC Infect Dis. 2019;19:1–9.
- Jones RM, Brosseau LM. Aerosol transmission of infectious disease. J Occup Environ Med. 2015;57:501–508.
- Cheung TMT, Yam LYC, So LKY, et al. Effectiveness of noninvasive positive pressure ventilation in the treatment of acute respiratory failure in severe acute respiratory syndrome. Chest. 2004;126:845–850.
- Han F, Jiang -Y-Y, Zheng J-H, et al. [Acute respiratory failure and noninvasive positive pressure ventilation treatment in patients with severe acute respiratory syndrome]. Zhonghua Jie He He Hu Xi Za Zhi. 2004;27:593–597.
- Bausewein C, Currow DC, Johnson MJ, editors. Palliative care in respiratory disease. Eur Respir Soc. 2016. DOI:10.1183/2312508X.erm7316