ABSTRACT
In 1960s, cromolyn sodium (CS) has been introduced as the first non-steroidal anti-inflammatory drug for the treatment of allergic and mast-cell driven diseases. Its applicability has been limited due to a poor bioavailability. Here we present pharmacokinetic data of a novel high concentration formulation of CS (PA101) delivered via a high-efficiency nebulizer (eFlow®) in healthy volunteers (HVs), allergic asthmatics and patients with indolent systemic mastocytosis (ISM). In HVs, PA101 40 mg and 80 mg (30 L) and PA101 40 mg (40 L), IntalTM (via LC® Plus) 20 mg and Nalcrom® (oral suspension) 200 mg showed maximum measured plasma concentration (Cmax) of 156, 236, 88.6, 17.8 and 5.23 ng/mL, respectively, with respective areas under the plasma time-concentration curve (AUC) of 338, 526, 212, 40.6 and 33.3 h·ng/mL. Systemic exposure (AUC) to CS with PA101 40 mg was approximately 8-fold and 11-fold higher compared to IntalTM and Nalcrom® in HVs, respectively. PA101 via eFlow® yielded comparable PK profiles in HVs and patients. Systemic bioavailability of PA101 was approximately 25% compared to approximately 1% for Nalcrom® and approximately 10% for IntalTM, respectively. These data warrant further research on the therapeutic potential of PA101 (via eFlow®) in allergic and mast-cell driven diseases.
Introduction
Since 1960s, cromolyn sodium (Disodium Cromoglycate, DSCG) has been widely used for the treatment of mast cell (MC)-mediated diseases, including allergic rhinoconjunctivitis, allergic skin disease, allergic asthma, food allergy and indolent systemic mastocytosis (ISM) [Citation1–Citation3]. Although its mechanism of action is still largely unknown, it was originally believed to be an MC modulator with pleotropic activity and dose-dependent effects. Cromolyn sodium indirectly blocks calcium from entering the cell resulting in stabilization of MCs, thus preventing the release of pro-inflammatory mediators and additionally inhibits the recruitment of inflammatory cells, including neutrophils, eosinophils and monocytes [Citation3]. Apart from its anti-allergic activity [Citation4,Citation5], several studies from the 1980s and 1990s report on bronchoprotective effects of cromolyn sodium against non-specific airway hyperresponsiveness and exercise-induced bronchoconstriction [Citation6,Citation7]. Further evidence showed that cromolyn sodium can reduce the cough reflex possibly through interference with the sensory nerves and their receptors [Citation8]. These observations may extend its therapeutic potential to conditions with chronic cough or cough hypersensitivity syndrome (CHS).
However, despite an excellent safety and tolerability, clinical applicability of cromoglycate sodium has been restricted by its overall poor pharmacokinetic profile following different administration routes [Citation9]. Previously approved and currently available formulations of cromolyn sodium (IntalTM, Gastrocrom® and Nalcrom®) achieved sub-optimal therapeutic concentrations, primarily due to suboptimal aerosol delivery with subsequent poor systemic exposure and bioavailability [Citation10,Citation11].
The rationale underlying the present studies consisted that improving the bioavailability by using a cromoglycate formulation in combination with a highly efficient nebulizer may increase the therapeutic applicability of cromolyn sodium in patients with allergic and/or mast-cell driven diseases such as asthma and ISM and potentially also in conditions with chronic cough as seen in (among others) idiopathic pulmonary fibrosis [Citation12] and advanced lung cancer [Citation13].
Subjects and methods
In a single-center study consisting of 3 parts, we investigated the pharmacokinetics (PK), and bioavailability of a solution of cromolyn sodium, PA101 (Patara Pharma, San Diego, CA, USA), delivered via eFlow® nebulizer (PARI, Grafelfing, Germany) in comparison with the currently marketed cromolyn formulations (Nalcrom® oral solution and IntalTM solution delivered via LC® Plus jet-nebulizer) in healthy volunteers (HVs), ISM patients and patients with mild allergic asthma. The explored dose levels of PA101 were anticipated to provide an improved efficacy compared to the highest doses of the marketed formulations of cromolyn sodium. Baseline demographics did not differ substantially across each study part. The study was conducted according to the Declaration of Helsinki for Medical Research involving Human subjects including locally applicable regulations. An Independent Ethics Committee (Stichting BEBO, Assen, the Netherlands) approved the study and all subjects gave written informed consent prior to participation.
Study medication
PA101 is a new formulation of a high concentration of sodium cromoglicate solution with osmolality and pH adjusted to a physiologically tolerable range (Patara Pharma, San Diego, CA, USA), delivered via a high-efficiency electronic nebulizer, eFlow® (PARI, Grafelfing, Germany) [Citation12]. The eFlow® is a hand-held, electronic nebulizer that uses a vibrating mesh membrane to generate aerosols with small(er) size particles, providing a more homogeneous deposition of the drug within the lungs compared to a general-purpose nebulizer [Citation14]. In this study, we used both the 30 L and the 40 L membranes (generating aerosol particles with a mass median aerodynamic diameter (MMAD) of 3.0 microns and 4.0 microns, respectively).
Nalcrom® oral solution is currently marketed for the treatment of food allergy and ISM.
IntalTM solution delivered via LC® Plus jet-nebulizer is currently marketed for the treatment of (mild) allergic asthma.
Study part 1 (healthy subjects, HV)
In part 1, twelve HVs (6 males/6 females; 19–38 years) received 6 single-dose treatments in a randomized, double-blind, 6-way cross-over design. Consecutive treatments were separated by a washout period of 2–5 days and consisted of: A) Placebo via eFlow®, B) PA101 40 mg via eFlow® with 30 L membrane, C) PA101 80 mg via eFlow® with 30 L membrane, D) PA101 40 mg via eFlow® with 40 L membrane, E) 20 mg IntalTM nebulizer solution, and F) 200 mg Nalcrom® oral solution.
Study part 2 and 3 (patients)
In part 2, five ISM patients (1 male/4 females; 35–65 years) received treatments B and F. In part 3, six patients with clinically stable, mild allergic asthma (2 males/4 females; 18–24 years; non-smokers, FEV1 77–112% of predicted value, infrequent use of short-acting beta2-agonists prn only) received treatments B and E. Parts 2 and 3 had a randomized, 2-way cross-over design with both treatments administered as single-day, three-times daily dosing. Washout period between consecutive treatments was 1–7 days. In all study parts, serial PK blood samples and urine collection were obtained up to 12 hours post-dosing and safety assessments were regularly performed.
Results
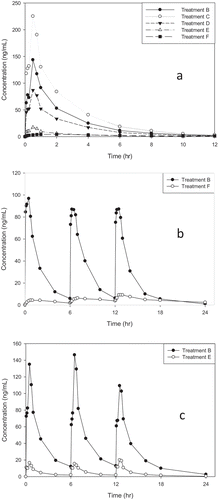
In Part 1 in HVs, PA101 40 mg with 30 L membrane generated 9- and 8-fold higher Cmax and AUC0-inf values, respectively, compared to IntalTM, and 31- and 10-fold higher Cmax and AUC0-inf values, respectively, compared to Nalcrom® (). PA101 80 mg (with 30 L membrane) further increased both Cmax and AUC0-inf by 1.5-fold compared to PA101 40 mg, indicating a near dose-proportionality. PA101 40 mg with 30 L membrane achieved 60% higher systemic exposure than PA101 40 mg with 40 L membrane, ()). In Parts 2 and 3, PA101PK parameters in patient groups were comparable to those in HVs (, ,c). Based on the total urine content of cromolyn, the systemic bioavailability of PA101 was approximately 25% compared to approximately 1% for Nalcrom® oral solution [Citation9] and approximately 10% for IntalTM nebulizer solution [Citation10]. Overall, all treatments were well-tolerated. No serious adverse events (AEs) occurred. Most AEs were of mild severity (n = 34), except for six AEs in Part 2 (5 moderate and 1 severe) and 2 AEs (both moderate) in Part 3 (). All AEs were resolved during the study. There were no clinically relevant findings for vital signs, ECG or laboratory safety data.
Table 1. Summary of pharmacokinetic parameters following administration of cromolyn sodium to healthy volunteers in study Part 1; in patients with indolent systemic mastocytosis (ISM) in study Part 2 and in patients with allergic asthma in study Part 3.
Table 2. Summary of adverse events recorded during study Part 1, Part 2 and Part 3.
Figure 1. Plasma cromolyn sodium concentrations and mean PK parameters.
Discussion
The new, high concentration cromolyn sodium formulation PA101 delivered via eFlow® nebulizer showed a substantially improved systemic bioavailability compared with the marketed products and was generally safe and well-tolerated in all study participants. Compared to IntalTM nebulizer solution and Nalcrom® oral solution, the systemic exposure was up to 8 and 11-fold respectively higher with PA101 40 mg inhaled via eFlow® nebulizer. In addition, the higher cromolyn sodium recovery in the urine after PA101 inhalation via eFlow® compared with the marketed formulations reflects a higher relative bioavailability in the lung tissue [Citation10].
Therapeutic efficacy of an inhaled product depends on its lung deposition which is primarily determined by particle size or droplet size, inhalation technique, nebulization time and delivery device [Citation11]. The eFlow® nebulizer is a high-efficiency nebulizer device with an innovative technology producing an adequate and homogenous lung deposition of orally inhaled solutions within a short period of time [Citation15]. A portable and easy to use drug-device combination allowing a short nebulizing time is anticipated to improve patient compliance, disease control and quality of life [Citation16].
The favorable pharmacokinetics of PA101 in combination with the eFlow® device in this study showed improvement in the bioavailability of cromolyn sodium compared to other formulations and devices as measured in plasma PK and urine excretion, regardless of administered doses. Although no pharmacodynamic parameters were tested in this intensive safety/PK study, the improved lung deposition and bioavailability of cromolyn sodium are anticipated to provide a superior protection against direct and indirect stimuli in asthma compared to the currently marketed formulations [Citation6]. Similarly, in patients with ISM, improved bioavailability may be of clinical benefit to the cutaneous, gastrointestinal and central-nervous-system manifestations of the disease.
The results from this study support the combination of PA101 with the eFlow® device to be further explored for the treatment of allergic, mast-cell driven and related disorders, including rhinoconjunctivitis, asthma, food allergy and mastocytosis, as well as in chronic cough associated with idiopathic pulmonary fibrosis [Citation12] and advanced lung cancer [Citation13].
Acknowledgments
The authors would like to thank all investigators and staff of the study centre involved in the studies for their effort in conducting these clinical trials.
Disclosure statement
The authors declare the following conflict of interest in relation to this work; K.A-E. and Z.D. are employees of QPS-Netherlands B.V., which received funding for the study conduct from Patara Pharma LLC, manufacturer of PA101 and various pharmaceutical companies for execution of phase 1 and 2 clinical trials. ZD report honoraria, consultancy and speaker fees from Astrazeneca, ALK, Aquilon, Boehringer Ingelheim, CSL, HAL Allergy, MSD, Sanofi-Genzyme. H.O-E is an employee of University Medical Centre Groningen, which received consultancy fees from Patara Pharma LLC for the study conduct. H.O-E report grants and personal fees for speaking from ALK-Abello, Novartis, MEDA pharma, and Chiesi and has received fees for organizing education from Novartis, MEDA Pharma, Mead Johnson, Chiesi, Sanofi and ALK-Abello and fees for consulting from Patara and BluePrint.
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
Notes on contributors
Khalid Abd-Elaziz
Khalid Abd-Elaziz clinical pharmacologist, associate medical director and clinical safety officer QPS-Netherlands.
Hanneke Oude Elberink
Hanneke Oude Elberink Allergist - Internist (Internist-allergologist) at University Medical Center Groningen.
Zuzana Diamant
Zuzana Diamant Professor Respiratory Allergy Research, Pulmonologist/Clin Pharmacologist, Phase 0-II studies, Netherlands.
References
- Howell JB, Altounyan RE. A double-blind trial of disodium cromoglycate in the treatment of allergic bronchial asthma. The Lancet. 1967 Sep 9;290(7515):539–6.
- Sinniah A, Yazid S, Flower RJ. The Anti-allergic Cromones: past, Present, and Future. Front Pharmacol. 2017;8:827.
- Murphy S. Cromolyn sodium: basic mechanisms and clinical usage. Drug Intell Clin Pharm. 1987 Jan;21(1 Pt 1):22–35.
- Shapiro GG, König P. Cromolyn sodium: a review. Pharmacotherapy. 1985 May-Jun;5(3):156–170.
- Brogden RN, Speight TM, Avery GS. Sodium cromoglycate (cromolyn sodium): a review of its mode of action, pharmacology, therapeutic efficacy and use. Drugs. 1974;7(3):164–282.
- Hoag JE, McFadden ER Jr. Long-term effect of cromolyn sodium on nonspecific bronchial hyperresponsiveness: a review. Ann Allergy. 1991 Jan;66(1):53–63.
- Morton AR, Ogle SL, Fitch KD. Effects of nedocromil sodium, cromolyn sodium, and a placebo in exercise-induced asthma. Ann Allergy. 1992 Feb;68(2):143–148.
- Dixon M, Jackson DM, Richards IM. The Action of Sodium Cromoglycate on ‘C’ Fibre Endings in the Dog Lung. Br J Pharmacol. 1980 Sep;70(1):11–13.
- Walker SR, Evans ME, Richards AJ, et al. The Fate of (14 C)disodium Cromoglycate in Man. J Pharm Pharmacol. 1972 Jul;24(7):525–531.
- Aswania OA, Corlett SA, Chrystyn H. Relative bioavailability of sodium cromoglycate to the lung following inhalation, using urinary excretion. Br J Clin Pharmacol. 1999 Jun;47(6):613–618.
- Keller M, Schierholz J. Have inadequate delivery systems hampered the clinical success of inhaled disodium cromoglycate? Time for reconsideration. Expert Opin Drug Deliv. 2011 Jan;8(1):1–17.
- Birring SS, Wijsenbeek MS, Agrawal S, et al. A novel formulation of inhaled sodium cromoglicate (PA101) in idiopathic pulmonary fibrosis and chronic cough: a randomised, double-blind, proof-of-concept, phase 2 trial. Lancet Respir Med. 2017 Oct;5(10):806–815.
- Moroni M, Porta C, Gualtieri G, et al. Inhaled Sodium Cromoglycate to Treat Cough in Advanced Lung Cancer Patients. Br J Cancer. 1996 Jul;74(2):309–311.
- Pham S, Ferguson GT, Kerwin E, et al. In Vitro Characterization of the eFlow Closed System Nebulizer With Glycopyrrolate Inhalation Solution. J Aerosol Med Pulm Drug Deliv. 2018 Jun;31(3):162–169.
- Knoch M, Keller M. The customized electronic nebulizer: a new category of liquid aerosol drug delivery systems. Exp Opin Drug Deliv. 2005;2:377–390.
- Sands D, Sapiejka E, Gąszczyk G, et al. T100 Study Group.Comparison of two tobramycin nebuliser solutions: pharmacokinetic, efficacy and safety profiles of T100 and TNS. J Cyst Fibros. 2014 Dec;13(6):653–660.
