ABSTRACT
Background: Fatigue is a common complaint in patients with idiopathic pulmonary fibrosis (IPF) and has been reported in a considerable percentage of patients. Fatigue is also a registered side effect of pirfenidone, one of two approved antifibrotic drugs. The Fatigue Assessment Scale (FAS) was developed for assessment of fatigue in sarcoidosis and validated in patients with sarcoidosis. FAS has been used in a few IPF studies but has not been validated.
Aims: To study the change in FAS after initiation of pirfenidone or nintedanib in the treatment of patients with IPF during a six-month period.
Methods: Between April 2017 and January 2018, all incident patients with IPF starting antifibrotic treatment were invited to complete FAS before, four weeks, three, and six months after initiation of antifibrotic treatment. Baseline characteristics including lung function were registered.
Results: Fifty-two patients were included, mean FVC% 84.8, mean DLCO% 51.4. Nintedanib was started in 25 patients; 27 patients started pirfenidone. Sixty-four percent of patients had a FAS score >22 indicating substantial fatigue at baseline. There was no statistically significant difference in FAS score for patients treated with nintedanib or pirfenidone at any time point. FAS score increased statistically significantly during the six-month follow-up. This change was driven by patients without substantial fatigue at baseline with an increase in FAS score of 8.4 points; patients with substantial fatigue at baseline experienced no statistically significant change.
Conclusion: A majority of patients with IPF suffered from substantial fatigue at the time of diagnosis. Fatigue progressed over time and increasing fatigue was associated with younger age, nintedanib treatment and low degree of fatigue at baseline. There was no significant difference in FAS score between the two antifibrotic treatments at any time point, even though fatigue is not a registered side effect in nintedanib.
Background
Idiopathic pulmonary fibrosis (IPF) is a chronic, progressive, and ultimately fatal fibrotic lung disease typically affecting elderly patients above the age of 60 [Citation1,Citation2]. IPF leads to progressive fibrosis of the lung parenchyma, resulting in an advancing loss of pulmonary compliance and a decline in gas exchange capacity [Citation3]. Patients have increasing dyspnea, dry cough, limited exercise capacity and often also fatigue. IPF is a rare disease with an estimated incidence of 3–9/100,000 and a median life expectancy between two and 5 years [Citation1,Citation2,Citation4]. Survival is improved in patients receiving one of the two registered antifibrotic drugs, pirfenidone, and nintedanib [Citation1,Citation2,Citation5,Citation6]
Fatigue is a central symptom in many diseases and a side effect of many medical treatments, including pirfenidone. In IPF, fatigue has been reported in up to 70% of patients [Citation1]. In the Ascend studies of pirfenidone, fatigue was reported by 21% of patients as a side effect, but there was no standardized measure of fatigue in the studies [Citation5]. Fatigue is not a registered side-effect to nintedanib.
Fatigue has been shown to have a substantial impact on patients’ self-care, depressive symptoms, and quality of life [Citation7,Citation8]; moreover, fatigue is a strong predictor of frailty, morbidity, and mortality [Citation9–11]. The cause of fatigue is unknown and possibly multifactorial [Citation12,Citation13]. Fatigue is impossible to quantify using physiological measures and is thus generally detected by means of patient reported outcome measures (PROMs) [Citation12]. Unfortunately, there is no validated, disease-specific PROM to measure fatigue in IPF.
The Fatigue Assessment Scale (FAS) was developed for assessment of fatigue in sarcoidosis [Citation14]. It consists of 10 items scored on a five-point Likert scale. Subsequently, a total score can be calculated. In sarcoid patients, FAS has proven to be valid and reliable to identify fatigue and a minimal clinically important difference has been estimated in sarcoidosis [Citation15]. The scale can be used in different patient populations [Citation16] and has been applied in IPF [Citation7,Citation16].
The aim of this study was to investigate the burden of fatigue measured by FAS at baseline in an incident cohort of patients with IPF and to examine the development of fatigue after initiation of treatment with either pirfenidone or nintedanib during six-month follow-up.
Methods
Between April 2017 and January 2018, all incident IPF patients starting antifibrotic treatment at the Center for Rare Lung Diseases, Department of Respiratory Diseases and Allergy, Aarhus University, Denmark, were invited to participate in the study.
Participants were eligible for inclusion, if they were above the age of 18, had a diagnosis of IPF based on the recent IPF diagnostic guidelines from 2011 [Citation17], i.e. no relevant exposure for the development of interstitial lung disease and a high-resolution computed tomography (HRCT) scan or a combination of HRCT and histopathology showing usual interstitial pneumonia pattern (UIP); antifibrotic treatment was initiated.
Baseline characteristics including age, gender, smoking history, type of antifibrotic drug, and comorbidities (diabetes, ischemic heart disease, kidney disease, stroke, prostate cancer, lymphoma, dementia, and sleep apnea) were registered. Measurement of forced vital capacity (FVC) and diffusing capacity of the lungs for carbon monoxide (DLCO) and a 6-min walk test (6MWT) were performed as part of the regular follow-up visit and results were registered.
The FAS questionnaire (Appendix 1) was completed by patients at baseline. The questionnaire was completed again at four weeks, three, and six months after initiation of antifibrotic treatment. After four weeks, FAS was completed by a nurse telephone interview (nurse telephone interviews are part of the standard follow-up program for IPF patients in antifibrotic treatment), whereas the questionnaire was completed at the regular visits at three and 6 months.
FAS is a self-reported questionnaire measuring fatigue, developed for, and validated in patients with sarcoidosis [Citation14]. FAS is a 10-item scale evaluating symptoms of chronic fatigue, each ranging from 1 (never) to 5 (always). Total scores can range from 10 to 50, and a FAS score >22 indicates substantial fatigue, while a FAS score ≥35 indicates extreme fatigue. In contrast to other similar measures, FAS considers fatigue as a unidimensional construct [Citation14].
The study was exempted from approval from the Central Denmark Region Committee of Health Research Ethics in accordance with national and international regulations due to the non-interventional design. Informed consent was obtained from participants before enrolment.
Descriptive data were presented as frequencies or means with standard deviation (SD) or 95% confidence intervals (CI). The Charlson comorbidity index was modified by adding sleep apnea (4 points) as a comorbidity in the analyses [Citation18]. Non-paired, two-sample t-tests and Fisher’s exact test were performed for cross-sectional comparisons. Linear regression was used to assess associations between fatigue and other variables at baseline. Correlations were assessed by Pearson’s coefficients. Partial correlations were analyzed to adjust for covariates. A mixed-effects model was fitted to analyze repeated measurements at various time points. At first, the model was fitted for the two treatment groups alone and afterward, covariates were added to adjust for the influence of other variables on fatigue. Normality was checked by QQ-plots. Models were checked by diagnostic plots of the residuals. A P < 0.05 was considered significant. STATA 14.2 (StataCorp, College Station, Texas) was used for statistical analyses.
Results
The FAS questionnaire was presented to a total of 52 patients. There were 59% males, mean age was 72.4 years (SD 8.4), 80% had been or were active smokers, and mean pack-years was 25.7 (SD 14.5). Twenty-five patients started treatment with nintedanib, and 27 patients started pirfenidone. During the observation period, five patients stopped their initial treatment due to unbearable side effects, and two of the five started on the other antifibrotic treatment. These patients were excluded from the analyses after termination of the initial treatment. Three additional patients died during the observation period. Twenty-four patients had different kinds of comorbidities at baseline, primarily sleep apnea (n = 6). The demographics are shown in .
Table 1. Baseline demographic data for all patients and patients treated with either pirfenidone or nintedanib. Data are presented as n or mean and standard deviation (SD)
Sixty-three percent reported a FAS score of more than 22, indicating that the majority of IPF patients suffered from substantial fatigue at enrolment. Mean FAS score was 24.3 (95% CI: 21.8 to 26.7) at baseline. There were no significant differences in FAS score between patients treated with pirfenidone or nintedanib at baseline, neither in the univariate (−1.7 (95% CI: −6.7 to 3.2), p = 0.49) nor the multivariate analysis (−0.03 (95% CI: −4.7 to 4.6), p = 0.99). Furthermore, no significant differences in FAS score were observed between males and females (3.9 (95% CI: −1.1 to 8.8), p = 0.12), although significantly more males that females reported a FAS score above 35, indicating extreme fatigue (p = 0.02).
At baseline, a univariate model including all patients showed a statistically significant relationship between FAS and comorbidities and an inverse relationship between the distance walked during the 6MWT. When adjusting for lung function, 6MWD, and comorbidities, increasing age also became significantly associated with decreasing FAS score. Lung function was not significantly associated with the FAS score (), neither was any of the other patient
Table 2. Linear regression analyses showing the associations between FAS score and relevant covariates
characteristics outlined in .
In the univariate model, total FAS increased significantly in the group receiving nintedanib during the six-month follow-up. However, in the group receiving pirfenidone, there was only a trend towards increasing FAS score (). Still, there was no significant difference between the two antifibrotic drugs, when comparing changes during 6 months. The results did not change substantially by adjustment for age, forced vital capacity (FVC), diffusing capacity for carbon monoxide (DLCO), and comorbidities ().
Table 3. Mixed-effects model of changes in FAS total during the six-month observation period
Only sleep apnea was significantly associated with the FAS score (1.9 (95% CI: 0.3 to 3.5, p = 0.02), while neither ischemic heart disease (−0.1 (95% CI: −3.0 to 3.0), p = 0.97) nor other comorbidities were associated with the FAS score (2.4 (95% CI: −0.7 to 5.4), p = 0.12). Raw and partial correlations between FAS total and comorbidities are presented below ().
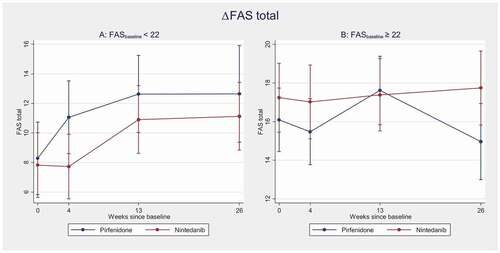
Figure 1. Change in FAS total during the six-month observation period in the two treatment groups, univariate model
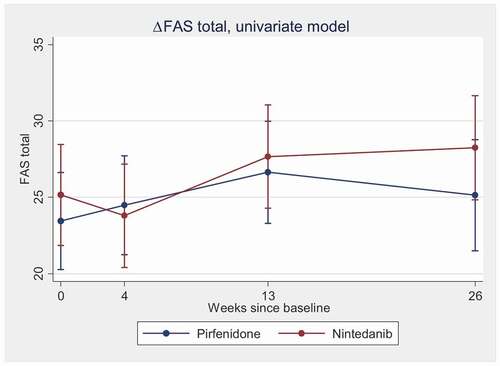
Table 4. Values in the lower left section are raw correlations, and values in the upper right section are partial correlation, adjusted for age, 6MWD and other comorbidities
After stratifying the patients according to substantial fatigue at baseline or not, there was no significant increase in FAS score during follow-up in the group with substantial fatigue at baseline (FAS≥22). On the other hand, there was a statistically significant increase in FAS over time in the group without substantial fatigue at baseline (FAS<22), without any significant difference between the two antifibrotic drugs (, ).
Discussion
To our knowledge, this study is the first to prospectively assess fatigue in a real-life cohort of patients with IPF treated with either pirfenidone or nintedanib. Our study followed patients with IPF over a period of 6 months and shows that patients with IPF suffer from a significant amount of fatigue at baseline, and fatigue is increasing over time independently of the type of antifibrotic treatment. Also, FAS increased significantly in the group receiving nintedanib, while only an increasing tendency was seen in the group receiving pirfenidone. These findings are not only statistically significant, but also clinically significant, since the difference is more than 10%, which is recognized as the minimally clinically important difference in FAS score in patients with sarcoidosis [Citation15].
Our findings show that many patients with IPF already suffered of a considerably fatigue before treatment, and that there is a trend towards worsening of fatigue over time. The increasing degree of fatigue was primarily driven by the patients not suffering from fatigue at baseline (FAS<22), whereas those with fatigue at baseline (FAS≥22) remained at a relatively stable level of fatigue. Since these results were not correlated to FVC or DLCO, fatigue seems not to be related to the severity of IPF. Instead, it might be a part of the disease, like fatigue in sarcoidosis where the degree of fatigue is not correlated to neither disease severity nor activity [Citation12]. Fatigue could also be due to a psychological change, caused by being appointed a diagnosis of a chronic life-threatening disease. This might mean that patients accept their physical limitations and stop trying to push these, thereby decreasing their physical activity and increasing fatigue. Unfortunately, this study did not include a quality of life questionnaire, and it is therefore not possible to investigate the relationship between quality of life and the increase in fatigue.
Fatigue has previously been examined using FAS in patients with sarcoidosis and IPF [Citation7,Citation16]. In the two studies, patients with sarcoidosis and IPF reported equally low FAS scores. This is possibly due to a lower DLCO in the patients with IPF, coursing them to more rapidly become exhausted and tired when performing any tasks. Unfortunately, DLCO was not measured in the study by Atkins et al. Nonetheless, it emphasizes the need for understanding the development of fatigue in patients with IPF and indicates that fatigue is equally important in IPF and in sarcoidosis.
Interestingly, our data show a statistically significant increase in FAS score in the group receiving nintedanib, independent of FAS score at baseline. This is interesting, since the increase is not found to the same extent in the pirfenidone group, and only pirfenidone has fatigue registered as a side effect. This might be because fatigue was not registered as an adverse event in the nintedanib studies [Citation19]. Hence, it has not been quantified to which extent the patients suffer from fatigue when treated with nintedanib. A possible explanation for the increased fatigue in this group compared with the pirfenidone group could be diarrhea, a very common side effect of pirfenidone. It could be speculated that the decreased absorption of energy due to diarrhea combined with constantly needing to go to the toilet drains energy, and thereby increase fatigue. Other side effects in the nintedanib arm, not perceived in the pirfenidone arm, could also be explanatory. Unfortunately, we did not register perceived side-effects, and was unable to investigate this further. Nevertheless, it should be investigated in a larger cohort.
As expected, increasing fatigue was associated with shorter 6MWD and the registered comorbidities. Further analyses into comorbidities revealed an association between sleep apnea and fatigue. This was expected, as sleep apnea is known to compromise the quality of sleep, and thus cause fatigue [Citation20]. No association was found between ischemic heart disease and fatigue.
Another notable result was the association between younger age and fatigue. This could be due to increased awareness of fatigue among younger patients that might be more likely to see fatigue as a problem. Older patients might be more likely to accept slowing down the pace due to an expected decrease in physical functioning during older age. Hence, they might experience fatigue to be a smaller problem. In accordance with this, studies have shown that general and physical fatigue decrease with age [Citation13,Citation21].
Another result of our study which diverges from the literature was that significantly more males than females reported extreme fatigue. Previous studies have shown that females are more likely to report higher levels of fatigue [Citation13,Citation21]. This could be due to the psychological state of mind. We hypothesized that those without psychological surplus experience more fatigue by the disease than those with a higher level of psychological resources. Since we have not measured the psychological state of our patients, we could not adjust for this. Perhaps these women are more likely to accept their condition, and make the necessary adjustments, while the men who are more physically active, perceive the limitations as worse.
One of the strengths of our study was the prospective design of incident patients who were examined before initiation of antifibrotic treatment and followed for 6 months. Measurements of disease progression (6MWT, FVC, and DLCO) were included and adjustment for comorbidities was performed. However, there are also some limitations. Only 52 participants were included, all from the same center and region. We cannot exclude that fatigue also depend upon cultural background and that a larger sample of the background population from other regions or countries would have shown different results. Educational level was not considered, and thus bias may have been introduced upon completion of the FAS questionnaire. We did not adjust our results for BMI or pack years, as our study population was too small. However, the impact of these factors is interesting and must be investigated, using a larger cohort.
Since FAS is not validated in IPF patients’ yet, it has therefore neither been investigated for reproducibility in this specific patient cohort. But FAS has been validated for lupus [Citation22] and stroke patients [Citation23], and the test–retest reliability is good in these studies. In a recent review of FAS, internal consistency in many other diseases including IPF patients was good [Citation16]. Therefore, we expect FAS to be similarly reliable also in this cohort.
In conclusion, a majority of patients with IPF suffered from substantial fatigue at the time of diagnosis. Fatigue progressed over time and the increasing fatigue was associated with younger age, nintedanib treatment and low degree of fatigue at baseline. There was no significant difference in FAS score between the two antifibrotic treatments at any time point, even though fatigue is not a registered side effect of nintedanib.
Contribution
EB conceived the idea and designed the study. VS and AA collected data, and LKA and TSP analyzed data. LKA, TSP and EB interpreted the data and wrote the manuscript; VS and AA critically reviewed the manuscript.
Acknowledgments
We thank all the patients contributing to this study and all the nurses who helped collect the FAS scores.
Disclosure statement
LKA reports no conflict of interest in or outside the study. EB and TP report no conflicts of interest related to the study. Outside the submitted work EB has received unrestricted grants, lecture fee, and participated in conference from Boehringer Ingelheim and Roche; TP has received: unrestricted grant and lecture fees from Boehringer Ingelheim and Roche.
Additional information
Notes on contributors
Line Kølner-Augustson
Line Kølner-Augustson, MD, is a medical doctor at Department of Respiratory Diseases and Allergy, Aarhus University Hospital, Denmark. Her main research focus is interstitial lung diseases.
Thomas Skovhus Prior
Thomas Skovhus Prior, MD, PhD, is a consultant at Department of Respiratory Diseases and Allergy, Aarhus University Hospital, Denmark. His main research focus is interstitial lung diseases.
Vibeke Skivild
Vibeke Skivild, is a nurse at Department of Respiratory Diseases and Allergy, Aarhus University Hospital, Denmark.
Anette Aalestrup
Anette Aalestrup, is a nurse at Department of Respiratory Diseases and Allergy, Aarhus University Hospital, Denmark.
Elisabeth Bendstrup
Elisabeth Bendstrup, MD, PhD, is a consultant at Department of Respiratory Diseases and Allergy, Aarhus University Hospital, Denmark. She is a specialist in pulmonary diseases and her main research focus is interstitial lung diseases.
References
- Guenther A, Krauss E, Tello S, et al. The European IPF registry (eurIPFreg): baseline characteristics and survival of patients with idiopathic pulmonary fibrosis. Respir Res. 2018;19(1). DOI:https://doi.org/10.1186/s12931-018-0845-5
- Jo HE, Glaspole I, Grainge C, et al. Baseline characteristics of idiopathic pulmonary fibrosis: analysis from the Australian idiopathic pulmonary fibrosis registry. Eur Respir J. 2017;49(2):1601592.
- Sgalla G, Iovene B, Calvello M, et al. Idiopathic pulmonary fibrosis: pathogenesis and management. Respir Res. 2018;19(1). DOI:https://doi.org/10.1186/s12931-018-0730-2
- Lee HE, Myong JP, Kim HR, et al. Incidence and prevalence of idiopathic interstitial pneumonia and idiopathic pulmonary fibrosis in Korea. Int J Tuberc Lung Dis. 2016;20(7):978–984.
- King TE, Bradford WZ, Castro-Bernardini S, et al. A Phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2083–8.
- Hughes G, Toellner H, Morris H, et al. Real world experiences: pirfenidone and nintedanib are effective and well tolerated treatments for idiopathic pulmonary fibrosis. J Clin Med. 2016;5(9):78.
- Atkins CP, Gilbert D, Brockwell C, et al. Fatigue in sarcoidosis and idiopathic pulmonary fibrosis: differences in character and severity between diseases. Sarcoidosis, Vasc Diffus Lung Dis off J WASOG. 2016;33(2):130–138.
- Mänty M, Rantanen T, Era P, et al. Fatigue and depressive symptoms in older people. J Appl Gerontol. 2014;33(4):505–514.
- Avlund K, Schultz-Larsen K, Davidsen M. Tiredness in daily activities at age 70 as a predictor of mortality during the next 10 years. J Clin Epidemiol. 1998;51(4):323–333.
- Ekmann A, Osler M, Avlund K. The predictive value of fatigue for nonfatal ischemic heart disease and all-cause mortality. Psychosom Med. 2012;74(5):464–470.
- Sheth JS, Xia M, Murray S, et al. Frailty and geriatric conditions in older patients with idiopathic pulmonary fibrosis. Respir Med. 2019;148:6–12.
- Drent M, Lower EE, De Vries J. Sarcoidosis-associated fatigue. Eur Respir J. 2012;40(1):255–263.
- Watt T, Groenvold M, Bjorner JB, et al. Fatigue in the Danish general population. Influence of sociodemographic factors and disease. J Epidemiol Community Health. 2000;54(11):827–833.
- De Vries J, Michielsen H, Van Heck GL, et al. Measuring fatigue in sarcoidosis: the Fatigue Assessment Scale (FAS). Br J Health Psychol. 2004;9(3). DOI:https://doi.org/10.1348/1359107041557048
- De Kleijn WPE, De Vries J, Wijnen PAHM, et al. Minimal (clinically) important differences for the Fatigue Assessment Scale in sarcoidosis. Respir Med. 2011;105(9):1388–1395.
- Hendriks C, Drent M, Elfferich M, et al. The Fatigue Assessment Scale: quality and availability in sarcoidosis and other diseases. Curr Opin Pulm Med. 2018;24(5). DOI:https://doi.org/10.1097/MCP.0000000000000496
- Raghu G, Collard HR, Egan JJ, et al. An Official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183(6):788–824.
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383.
- Richeldi L, Kreuter M, Selman M, et al. Long-term treatment of patients with idiopathic pulmonary fibrosis with nintedanib: results from the TOMORROW trial and its open-label extension. Thorax. 2018;73(6):581–583.
- Milioli G, Bosi M, Poletti V, et al. Sleep and respiratory sleep disorders in idiopathic pulmonary fibrosis. Sleep Med Rev. 2014;26. DOI:https://doi.org/10.1016/j.smrv.2015.03.005
- Tibblin G, Bengtsson C, Furunes B, et al. Symptoms by age and sex: the population studies of men and women in gothenburg, Sweden. Scand J Prim Health Care. 1990;8(1):9–17.
- Horisberger A, Courvoisier D, Ribi C. The Fatigue Assessment Scale as a simple and reliable tool in systemic lupus erythematosus: a cross-sectional study. Arthritis Res Ther. 2019;21(1). DOI:https://doi.org/10.1186/s13075-019-1864-4
- Bråndal A, Eriksson M, Wester P, et al. Reliability and validity of the Swedish Fatigue Assessment Scale when self-administered by persons with mild to moderate stroke. Top Stroke Rehabil. 2016;23(2):90–97.