ABSTRACT
Background: The prevalence of obstructive sleep apnoea syndrome (OSAS) keeps on rising. Daytime sleepiness resulting from fragmented sleep is the prime symptom, and obesity the major risk factor for OSAS. Quality of life with OSAS is often affected by depressive symptoms and anxiety. Nasal continuous positive airway pressure (CPAP) therapy reduces daytime sleepiness, but the results on the effect on mood, physical activity, and weight are controversial especially on long-term therapy. Purpose of this study was to evaluate these factors and predictors of weight gain during long-term CPAP therapy.
Methods: Consecutive patients (n = 223), referred to sleep study with suspected OSAS, were enrolled. Patients underwent a cardiorespiratory polygraphy at baseline and a battery of questionnaires was completed, both at baseline, and after three years of follow-up. Total of 149 (67%; M 65, F 84) patients completed the follow-up. Of the 149 patients, 76 (51.0%; M 32, F 44) used CPAP.
Results: In this study, depressive symptoms, anxiety, and sleepiness were alleviated during CPAP therapy. However, therapy did not have an influence on cravings of different food categories, or exercise habits and exercise duration. From the various factors studied, solely higher adherence to CPAP therapy was associated with weight gain.
Conclusions: This research provides further evidence that long-term CPAP therapy in patients with OSAS not only decreases sleepiness and improves sleep quality but could also alleviate depressive symptoms and anxiety. In addition, our study reinforces that CPAP therapy alone is not sufficient for weight management in patients with OSAS. Regardless of comprehensive battery of questionnaires, we were unable to establish markers predicting weight gain during therapy. We advise on life-style counselling and weight management program to all patients with obesity on CPAP therapy.
Introduction
Obesity is a major risk factor for obstructive sleep apnoea syndrome (OSAS). More than 70% of people with OSAS are overweight or obese [Citation1], and the prevalence estimates of OSAS in the middle-aged population are as high as 17% [Citation2]. In OSAS, pauses in breathing, due to collapse in airways during sleep, lead to fragmented sleep and nocturnal hypoxia, often resulting in depressive symptoms, anxiety [Citation3], and excessive daytime sleepiness (EDS) [Citation4]. In addition, obesity or depression per se, are risk factors for daytime sleepiness [Citation5]. Nasal continuous positive airway pressure (CPAP) therapy is currently the treatment of choice for OSAS [Citation6,Citation7]. Previous studies provide controversial results on the effect of CPAP therapy on depressive symptoms [Citation8], and the studies on the effect on anxiety are scarce [Citation9]. However, it has been suggested, that CPAP therapy alleviates anxiety particularly in women [Citation10].
CPAP therapy is known to reduce daytime sleepiness [Citation11], which could, in turn, improve diet, increase physical activity, and result in weight loss. The effect of CPAP therapy on energy balance is not fully known, but several mechanisms are likely. Currently, the best explanation is that when CPAP therapy is initiated, the sympathetic overdrive and the increased energy consumption caused by OSAS is diminished [Citation12], and therefore, unfortunately, CPAP therapy seems not to promote weight loss in most patients [Citation13]. Further, CPAP therapy does not seem to have an influence on diet or physical activity [Citation14], although in women a modest increase in recreational activity during CPAP treatment has been reported [Citation15]. Therefore, it has been suggested that active weight reduction programs should be combined to CPAP therapy [Citation16].
Our goal was to evaluate the effect of CPAP therapy on mood and sleepiness and to determine the factors that influence on weight during long-term CPAP therapy. We hypothesised, that in patients with OSAS, long-term adherence to CPAP therapy results in less depressive symptoms, less anxiety and sleepiness, improved sleep quality, decreased cravings to unhealthy food, and increased exercise duration and habits, but does not reduce weight. Further, we hypothesized, that not using CPAP for OSAS associates with weight gain without changes in mood, subjective sleep quality, cravings, or physical activity.
Subjects and methods
Baseline
The study population consisted of consecutive patients (n = 223, 54.7% women) referred to the Department of Pulmonary Diseases at Turku University Hospital, from March 2004 to October 2006, with symptoms suggesting OSAS, to rule out or confirm the diagnosis. All patients underwent a complete overnight in-hospital cardiorespiratory polygraphy (Embla®, Medcare Flaga hf, Medical Devices, Reykjavik, Iceland), their body mass index (BMI) was calculated based on measured height and weight, and after a standardised hospital meal, they completed a series of questionnaires around 6 PM to evaluate their current mood, sleep, exercise habits and duration, and cravings.
Cardiorespiratory polygraphy
The complete overnight in-hospital cardiorespiratory polygraphy included measurements of inspiratory flow pressure profile via nasal prongs, abdominal and thoracic movements, sleep position, periodic leg movements, electrocardiography, transcutaneous carbon dioxide partial pressure (PTcCO2; TCM3, Radiometer A/S, Copenhagen, Denmark), and arterial oxyhaemoglobin saturation (SaO2). A finger probe pulse oximeter (Oximeter Embla A10 XN, Embla, Denver, Colorado USA) was used to measure SaO2. From the SaO2 signals, episodes of arterial oxyhaemoglobin desaturation of 4% units or more per hour (oxygen desaturation index, ODI4), were automatically determined with Somnologica software. Artefacts were manually removed, and episodes of apnoea and hypopnea were determined visually by an experienced scorer, and expressed per hour (apnoea-hypopnea index, AHI) in bed from lights off to lights on, using internationally accepted criteria [Citation17]. Electroencephalogram was not included in the set-up, therefore respiratory effort-related arousals (RERA) were not scored.
OSAS was diagnosed if AHI was ≥5 per hour, since all the patients experienced classical symptoms suggesting OSAS. CPAP therapy was commenced if AHI was over 15 per hour. Moreover, if the patient suffered from severe symptoms disturbing daily life, such as excessive daytime sleepiness, insomnia, or mood disorders, CPAP therapy was introduced with AHI 5–15 per hour.
Questionnaires
EDS was evaluated with Epworth Sleepiness Scale (ESS, range 0–24, score >10 considered EDS) [Citation18]. Insomnia symptoms and sleep quality were evaluated with the help of the Pittsburgh Sleep Quality Index (PSQI, range 0–21) [Citation19], in which higher points indicate worse sleep quality. Self-reported usual sleep duration and sleep timing were recorded. Depression was screened with depression scale (DEPS, range 0–30, scores ≥9 suggesting depression) [Citation20]. Anxiety was evaluated with the State-Trait Anxiety Inventory (STAI, range 20–80) [Citation21], where the score was based on the feeling at the moment of filling the questionnaire, and higher scores indicating higher feeling of anxiety, and score over 38 indicating present anxiety. Visual analogue scales (VAS) were used to assess hunger, thirst, appetite, food quantities, and nausea [Citation22], and craving for different food categories, which included sweet, salty, starch, fruit, vegetables, meat/fish/egg, and dairies [Citation23]. Patients provided a score based on the feeling at the moment of completing the VAS scale (score 0–100 mm), the higher score indicating stronger craving. Exercise habits were evaluated with the question ‘How often do you exercise on an average?’. The answer alternatives were 1) not at all, 2) less frequently than once a week, 3) once a week, 4) twice a week, 5) three times a week, 6) four times a week, or 7) five times a week. Exercise duration was determined by the question: ‘How long is your exercise duration?’ Alternatives were. 1) 0–20 minutes, 2) 20–40 minutes, 3) 40–60 minutes and 4) over 60 minutes.
Follow-up study
Three years after the cardiorespiratory polygraphy was recorded, all the patients from the original cohort were invited for a follow-up visit, when questionnaires and measurements of BMI were repeated, but the cardiorespiratory polygraphy was not included. The original cohort was divided into CPAP users and non-users, according to their regular use of CPAP therapy. Patients, who still used CPAP therapy regularly (≥4 h/day), were considered as ‘users’, and those who did not commence or had discontinued their CPAP use, were considered ‘non-users’. As a standard treatment protocol, patients with CPAP therapy had follow-up visits once every year since CPAP initiation, when their CPAP pressure was checked, and average hours of use were documented with within-built clock counters, and if a patient did not meet the requirements of 4 hours daily use, the therapy was discontinued. Usage hours in clock counters, associated with the three-year follow-up point, were used in statistical analyses when comparing the change in usage hours from the beginning. Hours in clocks were available from the previous year.
At the end of the study,149 (66.8%; 65 men, 84 women) patients participated in the follow-up visit. Of the 149, 76 patients (51.0%; 32 men, 44 women) used CPAP. Among the 73 non-users (33 men, 40 women), there were 52 patients who refused CPAP treatment, seven patients who used it less than three months, five for 3–11 months, and nine for 12–24 months (). In non-users, there were 20 patients (14 men, 6 women) who did not have OSAS, as their AHI was <5/h, despite the classical symptoms of OSAS. The baseline characteristics of the study population are presented in . At baseline, there were missing values for PSQI in 4 (1.8%) patients, ESS in 6 (2.7%), appetite-VAS and hunger-VAS both in 27 (12%), and DEPS in 7 (3.1%) patients.
Table 1. Variables at baseline and differences between CPAP users and non-users
Figure 1. Formation of patient groups. From the original baseline cohort (n = 223), 74 patients did not participate in follow-up study three years later, when patients were divided into two groups (CPAP users or non-users). CPAP users had used CPAP device regularly over four hours per day for three years, and those who discontinued therapy before the three-year follow-up point, were treated as non-users, leaving 76 CPAP users and 73 non-users to final analysis
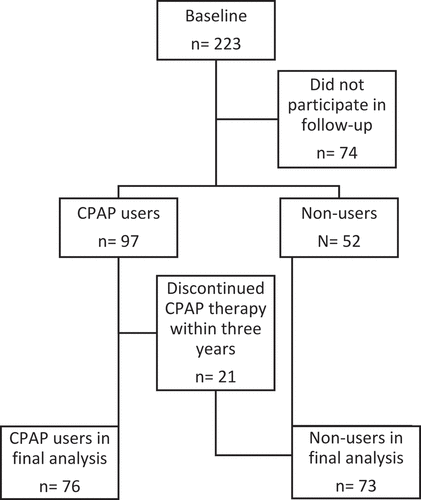
The main outcomes in this study were changes in depressive symptoms, anxiety, sleepiness, and weight.
Ethics
The study protocol was approved by the Ethics Committee of the Hospital District of Southwest Finland. Informed consent was obtained from all patients. All procedures performed were in accordance with the ethical standards of the Ethics Committee for the Hospital District of South West Finland and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Data analysis
Data are presented as median with range. All the analyses, except regression analyses, were analysed with SAS (Statistical software package for Windows version 9.2). Differences between the variables in CPAP users and non-users at baseline and at three-year follow-up point, and the differences of changes in variables during the follow-up period were evaluated with Mann-Whitney U-test for continuous variables and chi-squared test for categorical variables. Correlations between all the variables and the change in BMI were determined with Spearman’s rank correlation coefficients due to distribution of the data, also separately for the CPAP users and the non-users. To find predictors of weight change during the follow-up period among the CPAP users, all the variables that differed significantly between the CPAP users and the non-users at baseline were included in the regression analysis. Regression analysis was performed with ordinary least squares linear regression model with IBM SPSS version 25 (IBM Corp. Released 2017. IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp.). P-values less than 0.05 was considered statistically significant.
Results
Baseline
At baseline, the CPAP users had higher BMI and AHI compared to the non-users. The CPAP users also had more depressive symptoms and felt more thirst than the non-users (). Otherwise, the groups did not differ.
Three-year follow-up
BMI between the groups at the follow-up did not differ (35.3 kg/m2 vs. 29.4 kg/m2, p = 0.140), nor did the change in BMI (0.5 vs.0.3, p = 0.674). Moreover, change in self-reported sleep duration did not differ between the CPAP users and the non-users (0.24 hours/night vs 0.09 hours/night, p = 0.881). The median use of CPAP was 6.6 hours per day (range 1.4–11.3 hours) and median pressure 11 cmH2O (range 6–17 cmH2O). The change of variables after three years of follow-up are presented in .
Table 2. Change of variables during the 3-year follow-up and differences between CPAP-users and non-users
During the three-year follow-up, depressive symptoms decreased, and sleepiness and sleep quality improved more in the CPAP user group than in the non-user group. Anxiety decreased among the CPAP users whereas it increased slightly in non-users. Feeling of thirst did not change among the CPAP users but increased among the non-users. Otherwise, the groups did not differ.
Among the CPAP users, gaining weight during the follow-up period was associated with feeling of satiety after a full meal at baseline. Weight gain was not correlated with severity of OSAS, depressive symptoms, anxiety, sleep quality, exercise habits or duration, or craving for distinct food categories at baseline. Among the non-users, no associations between weight gain and the investigated variables at baseline were found (data not shown).
Moreover, weight gain in the CPAP users was associated with higher use of CPAP and craving less sweets at the three-year follow-up. Among the non-users, weight gain was associated with craving dairy products at the follow-up. Weight gain was not associated with other variables ().
Table 3. Correlations between the change in BMI and variables at the 3-year follow-up
Regression analysis
In regression analysis, to determine the factors that influenced on weight, and were different between the CPAP users and the non-users, all the variables that differed significantly between the CPAP users and the non-users, were included, consisting of baseline BMI, baseline AHI, baseline ODI4, baseline depressive symptoms, and baseline thirst. From these, only baseline BMI and depressive symptoms at baseline were associated with weight gain among the whole study population (). Further, when the CPAP users and the non-users were analysed separately, the results did not change. However, when the CPAP users were divided according to gender, the effect of depressive symptoms disappeared in men. In non-user men, the results remained. Among the non-user women, depressive symptoms was not a significant factor influencing on weight gain, whereas baseline BMI was (). Other variables did not have an effect.
Table 4. Regression analysis of subgroups and significant factors that effect on weight gain
Discussion
This three-year prospective follow-up study showed that treating OSAS patients with CPAP therapy alleviated depressive symptoms, anxiety, and sleepiness as expected [Citation24]. On the other hand, anxiety slightly increased among the CPAP non-users. In addition, study provided further evidence that weight gain among patients with OSAS associates with higher long-term adherence to CPAP therapy. Moreover, unexpectedly, craving less sweets at the three-year follow-up was associated with weight gain in CPAP user group. Weight gain was not associated with anxiety, improved sleepiness or sleep quality, or exercise habits and duration, or other cravings.
Depressive symptoms and depressive disorder are known to have a strong association with obstructive sleep apnoea [Citation8]. In one of the first studies in this field, 24% of 25 male OSAS patients had depressive or anxiety symptoms [Citation25]. Further, it has been reported, that women with OSAS have more depressive symptoms than men [Citation26], but most of the previous studies have consisted mainly of men [Citation27]. Our study had 46% of women, therefore being gender-balanced. Depressive symptoms overlap with OSAS symptoms. Therefore, treatment response to CPAP may reveal depression underlying OSAS. We propose that part of the depressive symptoms at baseline were secondary to the untreated OSAS, whereas the residual depressive symptoms observed at follow-up could have another aetiology. Previous studies provide limited and contradictory data on the effect of CPAP therapy on depressive symptoms, especially during long-term therapy [Citation8]. In one study, CPAP therapy for a few months alleviated depressive symptoms [Citation28], whereas in another study no effect was found [Citation29]. It is unlikely that reduction of depressive symptoms after three years on CPAP observed in our study was due to a placebo-response [Citation30].
Anxiety is a common symptom in OSAS, with a reported prevalence as high as 53.9% [Citation31]. Data on how anxiety responds to CPAP is scarce. Our results, derived from a cohort with a strong female representation, are in line with an earlier study, according to which CPAP therapy alleviates anxiety particularly in women [Citation10]. In our study, the level of anxiety, similar at baseline, started to deviate during the three-year follow-up, depending on whether OSAS was treated or not. Since sleep studies were not repeated, we were not able to assess, whether the increased anxiety in the untreated group would have been associated with aggravation of their sleep-disordered breathing during the follow-up period. One could also argue, that despite similar level of reported anxiety at baseline, the ones choosing not to be treated could be those with a tendency to respond with anxiety to various stresses, such as wearing a mask at night. This tendency to anxiety could have explained the non-adherence to CPAP, as well as the increased anxiety observed over time. The tools we used to assess the effect of CPAP therapy on depressive symptoms (DEPS), or anxiety (STAIS), are standard questionnaires, used to screen the corresponding diseases. Although statistically significant, one can question, whether the magnitude of these changes have biological significance. The observed changes should be interpreted as trends in the degree of depressive or anxiety symptoms, without any relevance to depression or anxiety as diseases.
Sleepiness and sleep quality improved among CPAP users in our study, which is in line with previous studies. Improved sleep quality in our study could explain the reduction of sleepiness. In 2003, Patel et al. stated in their meta-analysis, that CPAP therapy alleviates sleepiness in patients with OSA in a diverse range of populations. However, only three of the studies included in the analysis, had less than 75% of men. It has been previously shown, that women report excess daytime sleepiness more frequently than men [Citation31] and that 12 weeks of CPAP therapy reduces the self-reported sleepiness in women [Citation10]. Our study confirms this finding in long-term CPAP therapy.
CPAP users felt more thirst at baseline. However, only two of the CPAP users had a diagnosed diabetes at baseline, and their fasting blood glucose did not differ from those of the non-users (data not shown). Elevated levels of the cardiac natriuretic peptides (ANP, BNP) in patients with OSAS, particularly those with hypertension, could play a role in feeling thirst when untreated. However, other mechanisms are likely, since CPAP therapy effectively reduces ANP and BNP levels [Citation32] but feeling thirst did not regress with long-term adherence to CPAP. Feeling of thirst in the morning, can also result from snoring and mouth breathing, but is not usually the case later during the day, and may not explain our results. Therefore, other mechanisms inducing thirst must be involved.
Association of weight gain and satiety among CPAP users might simply imply, that they ate more than those not gaining weight. An alternative explanation could be that CPAP users gain weight, because the therapy reduces the resting metabolic rate [Citation16]. Unexpectedly, weight gain was not associated with craving more sweet or high-carbohydrate products. This might be explained by the unfortune fact, that the questionnaires were introduced to the patients right after a full meal, when cravings are usually absent. Severity of OSAS, anxiety, sleepiness or sleep quality, or exercise habits, or cravings were not associated with weight gain in our study. In regression analysis, the only factors influencing on weight were depressive symptoms and BMI at the beginning, and the results remained when the CPAP users and non-users were evaluated separately. It would be important to identify those OSAS patients who are going to gain weight, already when CPAP therapy is initiated. However, even using an extensive set of questionnaires, as well as cardiorespiratory polygraphy reports, we were unable to establish markers predicting weight gain during therapy. Of note, the CPAP users had higher BMI in the beginning, but the change in BMI did not differ between the CPAP users and the non-users. Moreover, the change in BMI was indeed minor in both groups during the follow-up period, and most likely without clinical significance.
The strength of our study is a gender-balanced, modest size, prospective clinical cohort, with relatively long follow-up period. Previous studies have consisted mainly of men, except the one recruiting only women [Citation10]. Moreover, we utilized a comprehensive battery of questionnaires to evaluate possible association with weight gain. However, some limitations to our study must be considered. Our questionnaires were introduced in the evening, right after a full meal. This influences on mood and cravings and could alter the results compared to the questionnaires completed in the morning. We did not use food diaries which might provide a deeper insight into the issues related to weight gain than questionnaires administered at a single point at baseline and follow-up. In addition, sub-analyses should be interpreted with caution, due to a small number of patients in gender subgroups.
Conclusion
Our study provides further evidence, that long-term CPAP therapy in patients with OSAS might not only alleviate depressive symptoms and anxiety, but also decrease sleepiness. In addition, our study emphasises previous expectations, that weight continues to increase during long-term adherence to CPAP therapy, irrespectively of changes in cravings or exercise habits. Further studies are needed to establish biomarkers identifying the subset of patients at risk of further weight gain during CPAP therapy and in need of preventive measures.
Disclosure statement
Dr. Aro reports participation in international scientific conferences sponsored by Boehringer-Ingelheim and Takeda. Dr. Anttalainen reports personal Fees paid for lectures from the Finnish Medical Association, ResMed Finland, Roche, Mundipharma and Fisher & Paykel. Participation for the international scientific conferences sponsored by Boehringer-Ingelheim and Roche. Member of the Finnish Current Care Task Force for Adult obstructive sleep apnoea and employed by the Turku University Hospital. Dr Polo has no conflicts of interest. Dr. Saaresranta reports speaking fees from Medical Association of Duodecim, City of Turku, Association of Finnish Respiratory Physicians, ResMed Finland, Boehringer Ingelheim, Mundiphrama, Chiesi and AGA Health Care, registration fee, hotel and travel costs to participate in international scientific congresses paid in full or partly by European Respiratory Society, Nordic Lung Congress 2017 and 2019, Roche, Boehringer Ingelheim, and Chiesi. President of the Task Force for Finnish Current Care Guidelines of Adult OSA.
No potential conflict of interest was reported by the authors.
Additional information
Funding
Notes on contributors
Miia M. Aro
Miia Aro is a consultant in Respiratory Medicine and Allergology and working as a Chief Medical Advisor in Finnish Lung Health Association and the main focus of her research is sleep disordered sleeping.
Ulla Anttalainen
Ulla Anttalainen, PhD, associate professor, is a consultant in Respiratory medicine and working as a chief specialist in department of Pulmonology and Clinical Allergology in Turku University Hospital and her research focuses mainly on sleep disordered breathing.
Olli Polo
Olli Polo, phD, is a consultant in Respiratory Medicine and his research focuses also on sleep medicine.
Tarja Saaresranta
Tarja Saaresranta, PhD, professor is a consultant in Respiratory Medicine and working as a chief specialist in the department of Pulmonology and Clinical Allergology in Turku University Hospital and her research covers widely sleep disordered breathing and is conducted also in Department of Physiology, Sleep Research Unit in Turku University.
References
- Malhotra A, White DP. Obstructive sleep apnoea. Lancet. 2002;360(9328):237–8.
- Peppard PE, Young T, Barnet JH, et al. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–1014.
- Saunamäki T, Jehkonen M. Depression and anxiety in obstructive sleep apnea syndrome: a review. Acta Neurol Scand. 2007;116(5):277–288.
- Bonnet MH, Arand DL. Clinical effects of sleep fragmentation versus sleep deprivation. Sleep Med Rev. 2003;7(4):297–310.
- Bixler EO, Vgontzas AN, Lin H-M. Excessive daytime sleepiness in a general population sample: the role of sleep apnea, age, obesity, diabetes, and depression. J Clin Endocrinol Metab. 2005;90(8):4510–4515.
- Polo O, Berthon-Jones M, Sullivan CE, et al. Management of obstructive sleep apnoea/hypopnoea syndrome. Lancet. 1994;344(8923):656–660.
- Sullivan CE, Issa FG, Berthon-Jones M, et al. Reversal of obstructive sleep apnoea by continuous positive airway pressure applied through the nares. Lancet. 1981;1(8225):862–865.
- Hobzova M, Prasko J, Vanek J, et al. Depression and obstructive sleep apnea. Neuroendocrinol Lett. 2017;38:343–352.
- Zheng D, Xu Y, You S, et al. Effects of continuous positive airway pressure on depression and anxiety symptoms in patients with obstructive sleep apnoea: results from the sleep apnoea cardiovascular endpoint randomised trial and meta-analysis. EClinicalMedicine. 2019;11:89–96.
- Campos-Rodriguez F, Queipo-Corona C, Carmona-Bernal C, et al. Continuous positive airway pressure improves quality of life in women with obstructive sleep apnea. a randomized controlled trial. Am J Respir Crit Care Med. 2016;194(10):1286–1294.
- Avlonitou E, Kapsimalis F, Varouchakis G, et al. Adherence to CPAP therapy improves quality of life and reduces symptoms among obstructive sleep apnea syndrome patients. Sleep Breath. 2012;16(2):563–569.
- Bamberga M, Rizzi M, Gadaleta F, et al. Relationship between energy expenditure, physical activity and weight loss during CPAP treatment in obese OSA subjects. Respir Med. 2015;109(4):540–545.
- Hoyos C, Killick R, Yee B, et al. Cardiometabolic changes after continuous positive airway pressure for obstructive sleep apnoea: a randomised sham-controlled study. Thorax. 2012;67(12):1081–1089.
- Shechter A. Effects of continuous positive airway pressure on energy balance regulation: a systematic review. Eur Respir J. 2016;48(6):1640–1657.
- Batool-Anwar S, Goodwin JL, Drescher AA, et al. Impact of CPAP on activity patterns and diet in patients with Obstructive Sleep Apnea (OSA). J Clin Sleep Med. 2014;10(5):465–472.
- Drager LF, Brunoni AR, Jenner R, et al. Effects of CPAP on body weight in patients with obstructive sleep apnoea: a meta-analysis of randomised trials. Thorax. 2015;70(3):258–264.
- Iber C, Ancoli-Israel S, Chesson AJ, et al. American academy of sleep medicine; 2007. The AASM manual for the scoring of sleep and associated events: rules, terminology, and technical specifications. 1st Ed.: Westchester, Illinois: American Academy of Sleep Medicine, 2007.
- Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. J Sleep Res Med. 1991;14:540–545.
- Buysse DJ, Reynolds CF, Monk TH, et al. The Pittsburgh sleep quality index (PSQI): A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213.
- Salokangas RK, Poutanen O, Stengard E. Screening for depression in primary care development and validation of the depression scale, a screening instrument for depression. Acta Psychiatr Scand. 1995;92(1):10–16.
- Spielberger CD, Gorsuch RL, Lushene PR, et al. Manual for the state-trait anxiety inventory (Form Y). Palo Alto, CA: Consulting Psychologists Press. 1983. DOI:https://doi.org/10.1007/978-1-4419-9893-4.
- Stubbs RJ, Hughes DA, Johnstone AM, et al. The use of visual analogue scales to assess motivation to eat in human subjects: a review of their reliability and validity with an evaluation of new hand-held computerized systems for temporal tracking of appetite ratings. Br J Nutr. 2000;84(4):405–415.
- Parker BA, Sturm K, MacIntosh CG, et al. Relation between food intake and visual analogue scale ratings of appetite and other sensations in healthy older and young subjects. Eur J Clin Nutr. 2004;58(2):212–218.
- Gupta MA, Simpson FC. Obstructive sleep apnea and psychiatric disorders: a systematic review. J Clin Sleep Med. 2015;11(2):165–175.
- Ramar K, Guilleminault C. Excessive daytime sleepiness and obstructive sleep apnea syndrome. Sleep Med Clin. 2006;1(1):63–78.
- Pillar G, Lavie P. Psychiatric symptoms in sleep apnea syndrome: effects of gender and respiratory disturbance index. Chest. 1998;114(3):697–703.
- McMahon JP, Foresman BH, Chisholm RC. The influence of CPAP on the neurobehavioral performance of patients with obstructive sleep apnea hypopnea syndrome: a systematic review. WMJ. 2003;102(1):36–43.
- Sánchez AI, Buela-Casal G, Bermúdez MP, et al. The effects of continuous positive air pressure treatment on anxiety and depression levels in apnea patients. Psychiatry Clin Neurosci. 2001;55(6):641–646.
- Muñoz A, Mayoralas LR, Barbé F, et al. Long-term effects of CPAP on daytime functioning in patients with sleep apnoea syndrome. Eur Respir J. 2000;15(4):676–681.
- Yu BH, Ancoli-Israel S, Dimsdale JE. Effect of CPAP treatment on mood states in patients with sleep apnea. J Psychiatr Res. 1999;33(5):427–432.
- Rezaeitalab F, Moharrari F, Saberi S, et al. The correlation of anxiety and depression with obstructive sleep apnea syndrome. J Res Med Sci. 2014;19(3):205–210.
- Kita H, Ohi M, Chin K, et al. The nocturnal secretion of cardiac natriuretic peptides during obstructive sleep apnoea and its response to therapy with nasal continuous positive airway pressure. J Sleep Res. 1998;7(3):199–207.
