ABSTRACT
Background: Allergen avoidance is important in allergic asthma management. Nocturnal treatment with Temperature-controlled Laminar Airflow (TLA) has been shown to provide a significant reduction in the exposure to allergens in the breathing zone, leading to a long-term reduction in airway inflammation and improvement in Quality of life (QoL). Allergic asthma patients symptomatic on Global Initiative for Asthma (GINA) step 4/5 were found to benefit the most as measured by Asthma Quality of Life Questionnaire (AQLQ). However, the effect of TLA on severe asthma exacerbations is uncertain and therefore a meta-analysis was performed.
Methods: Patients with severe allergic asthma (GINA 4/5) were extracted from two 1-year randomised, double-blind, placebo-controlled trials conducted with TLA. A meta-analysis of the effect on severe exacerbations was performed by negative binomial regression in a sequential manner, defined by baseline markers of asthma control (symptoms and QoL scores).
Results: The pooled dataset included 364patients. Patients with more symptoms at baseline (ACT<18 or ACQ7>3; N=179), had a significant mean 41% reduction in severe exacerbations (RR=0.59 (0.38-0.90); p=0.015) in favour of TLA. Higher ACQ7 cut-points of 3.5-4.5 resulted in significant reductions of 48-59%.More uncontrolled patients based on AQLQ total and symptom domains ≤3.0 at baseline also showed a significant reduction in severe exacerbations for TLA vs. placebo ((47% (p=0.037) and 53% (p=0.011), respectively). The meta-analysis also confirmed a significant difference in AQLQ-responders ((Minimal Clinically Important Difference)≥0.5; 74% vs. 43%, p=0.04).
Conclusion: This meta-analysis of individual patient data shows a beneficial effect on severe exacerbations and quality of life for TLA over placebo in more symptomatic patients with severe allergic asthma. These outcomes support the national management recommendations for patients with symptomatic severe allergic asthma. The actual effect of TLA on severe exacerbations should be confirmed in a prospective study with larger numbers of patients.
Introduction
Treatment alternatives for patients with symptomatic severe asthma include high-dose inhaled corticosteroids (ICS) plus a bronchodilator (e.g. long-acting β2-agonists and/or muscarinic antagonists) and leukotriene receptor antagonists according to Global Initiative for Asthma (GINA) step 4/5 [Citation1]. Alternatives for add-on treatment for such patients include systemic corticosteroids, anti-immunoglobulin E treatments and monoclonal antibody inhibitors of interleukins 4, 5, and 13 [Citation2–4]. All treatment alternatives are effective, with long-term safety favouring the biological agents, though post-market experience differs, and the costs of biological treatments are very high [Citation5,Citation6]. A concurrent treatment option for severe asthma which has received little attention is allergen avoidance. One such method involves nocturnal temperature-controlled laminar airflow (TLA). TLA reduces exposure to allergens and irritant particles during sleep by delivering filtered and slightly cooled air to the breathing zone of the patient [Citation7,Citation8]. A double-blind, placebo-controlled one-year trial in patients with inadequately controlled persistent allergen-driven asthma by Boyle et al. (the 4A asthma trial) [Citation9] demonstrated a significant difference in Asthma Quality of Life Questionnaire (AQLQ) responder rate for TLA compared to placebo. The effect of TLA increased in the pre-defined subgroups with more symptomatic and severe asthma [Citation9,Citation10]. In an open-label study, TLA was shown to reduce severe exacerbations [Citation11]. Reductions in airway inflammation by TLA as shown by reductions in exhaled nitric oxide levels (FeNO) have also been reported [Citation9,Citation12]. TLA has shown cost-effectiveness at ranges consistent with the National Institute for Health and Care Excellence (NICE) standards [Citation8,Citation13]. These data have led to TLA being recommended for asthma patients with symptomatic severe allergic asthma as stated by NICE, the Scottish, and the Swedish guidelines [Citation14,Citation15].
In the 4A asthma trial, which was not powered to investigate an effect on exacerbations, no effect could be demonstrated on severe exacerbations, when all patients were included (annual exacerbation rate: 0.17 for TLA vs 0.24 for placebo; rate ratio (RR) = 0.71; p = 0.5) [Citation7]. However, in the pre-defined subgroups, increased reduction in exacerbation rates was observed when more symptomatic and more severe patients were analyzed separately. For patients with symptomatic severe asthma defined as GINA 4/5 and Asthma Control Test (ACT)<18 (n = 87), a 59% reduction of severe exacerbations was demonstrated (annual exacerbation rate: 0.23 for TLA vs 0.57 for placebo; RR = 0.41; p = 0.07) [Citation7,Citation9]. Recently, a one-year study (LASER) by Kapoor et al. [Citation16] in patients treated according to GINA step 4/5 showed no significant effect on severe exacerbations (RR = 0.92; p = 0.62) between TLA and placebo. The study demonstrated a high placebo response, especially in the patients with better symptom control, while the difference in effect between TLA and placebo descriptively increased for patients with more symptoms.
Our main objective was to investigate the effect of TLA on severe exacerbations among patients with more symptomatic severe allergic asthma when the two 1-year studies were pooled into a dataset for a meta-analysis of individual patient data. ACQ + ACT, symptom domain of AQLQ and total AQLQ were used as baseline cut-offs to extract exacerbation-prone patients. The secondary objective was to confirm the effect on the responder rate ratio for AQLQ, as demonstrated in the 4A asthma trial [Citation9].
Materials and methods
The current study is a post-hoc analysis of data from the LASER trial (Kapoor et al., ClinicalTrials.gov NCT02813811) [Citation16] and the 4A asthma trial (Boyle et al., ClinicalTrials.gov NCT00986323) [Citation9]. In the following text, the LASER trial will be referred to as Study A and the full 4A asthma trial as Study B1. The subset of patients with symptomatic severe allergic asthma (i.e. only GINA 4/5) from the 4A asthma trial will be referred to as Study B2.
Ethics
Kapoor et al.: The study was approved by Health Research Authority: NRES Committee South Central – Berkshire REC Reference 14/SC/0092, IRAS Project ID 148,386, and written informed consent was obtained from all patients. Boyle et al.: The study was approved by responsible institutional review boards in each participating centre and written informed consent was obtained from all patients/parents. The studies complied with the Declaration of Helsinki.
Patients
In Study A, patients with a clinical diagnosis of asthma were aged 16–75 years, treated according to GINA 4/5, severe asthma defined by requirement for high-dose ICS plus a second controller and/or systemic corticosteroids, poorly controlled asthma demonstrated by two or more severe asthma exacerbations, requiring systemic corticosteroids in the preceding 12 months plus an Asthma Control Questionnaire (ACQ)7 score >1, and atopic status, defined as sensitisation to ≥1 perennial indoor aeroallergens [Citation16]. In Study B1, patients with physician-diagnosed asthma were aged 7–70 years, with asthma treated according to GINA 2–4/5, defined by daily use of inhaled corticosteroids (ICS) for the last 6 months, poorly controlled asthma demonstrated by AQLQ-score ≤5.5 at inclusion, and atopic status, defined by sensitisation to a pet allergen (cat and/or dog) and/or house dust mite.
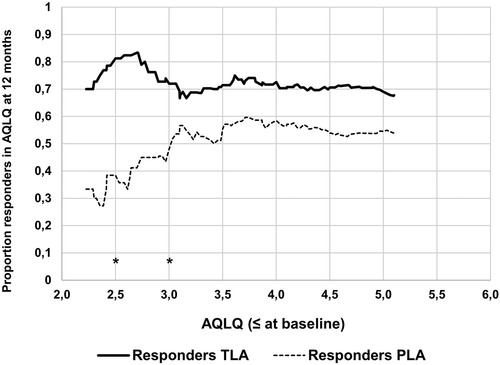
In the current study (Study A+ B2), a pooled dataset with individual patient data from the two studies is presented (), including all patients with severe allergic asthma treated according to GINA step 4/5 from both studies.
Study design
The original studies, Study A and Study B1 [Citation9,Citation16] were both phase III multicentre, double-blind, placebo-controlled, parallel-group trials in which patients were randomised to receive add-on treatment with TLA (Airsonett AIR4, Airsonett AB, Ängelholm; Sweden) or a placebo device for 1 year. In Study A and B1 all participants were evaluated during the study at baseline and after 3, 6, 9, and 12 months, and via completion of a diary. The randomization ratio was 1:1 in Study A and 2 (TLA):1 (placebo) in Study B1.
Preparation of the pooled dataset
The final analysis datasets from Study A and Study B1 were retrieved. Patients from Study B1 not in GINA 4/5 were removed. Baseline demography, lung function, ACQ7 (from study A), ACT (from study B2), AQLQ-scores, and severe exacerbations were extracted into the pooled dataset, as they were collected and data-locked in the respective study. Both studies defined severe exacerbations in accordance with the American Thoracic Society/European Respiratory Society (ATS/ERS) guidelines [Citation17]. For further details regarding the collection of severe exacerbations, see the papers by Boyle et al. [Citation9] and Kapoor et al. [Citation16].
Outcome measures
In Study A, the primary outcome was the frequency of severe asthma exacerbations occurring over a 12-month period. In Study B1 the primary outcome measure was the response rate in QoL assessed by Mini AQLQ or in children 7–11 years, the Paediatric AQLQ (PAQLQ), and in the following text, the term AQLQ has been used in the combined mini-AQLQ and PAQLQ dataset. Severe exacerbations were recorded as a secondary outcome. In addition, three pre-specified sub-groups were also analysed: ACT score <18; GINA 4/5; ACT score <18 plus GINA 4/5.
In the current study, the primary objective was to assess whether TLA is effective in reducing severe exacerbations in patients with more symptomatic severe allergic asthma. The primary outcome on severe exacerbations in symptomatic patients was performed using baseline ACQ in Study A and baseline ACT in Study B1 as cut points, due to the use of different symptom questionnaires in the two studies. The two baseline symptom questionnaires were used separately (not combined) per study to extract exacerbation-prone patients. This analysis was complemented by using the baseline symptom domain in AQLQ and baseline total AQLQ, using total AQLQ as the cut point. The secondary objective was to confirm the effect on the responder rate ratio for total AQLQ, using baseline total AQLQ as cut point, as demonstrated in Study B1.
Statistical analyses
The data were summarised and analysed by similar methods as previously used for the two studies, a negative binomial regression for the number of severe exacerbations, and logistic regression of responders for AQLQ. For the meta-analysis of individual patient data, treatment and study were fixed factors. With study as a fixed factor in the model, the pooled results are approximately weighted by study size. Baseline AQLQ was used as a covariate when analysing AQLQ. No covariates were used for the analysis of number of severe exacerbations.
To describe the data across the range of the baseline values a cumulative approach was used where data were averaged stepwise up to each of the ordered unique values of the particular baseline variable. A significant effect on the number of severe exacerbations was determined using sequential analysis with baseline scores increasing or decreasing with 0.5 unit scores until no significant difference could be demonstrated.
ACQ was reduced as described above, while ACT<18 was used as a fixed cut-point because this symptom severity cut-point was pre-specified in Study B1. Evidence has been identified for a relationship between ACT score and ACQ score [Citation18], and both scores have been shown to correctly predict GINA-defined uncontrolled asthma [Citation19]. Due to low patient numbers, the data were not analysed at the extreme of severity. In this post-hoc analysis statistical significance level was 5% (two-sided) and no overall adjustment for multiple testing was employed.
Results
Patient populations
The population for the primary analysis in Study A and Study B1 included 240 and 282 patients, respectively. For further details and patient flow in the respective study, see [Citation9] and [Citation16].
presents baseline demographics for the population extracted in the current study. From study A, all patients for whom data on AQLQ were available were included (N = 235). From study B2 all patients in GINA 4/5 were included (N = 129) (). The pooled dataset from Study A and Study B2 thus included 364 patients. Within each of the studies, the TLA and placebo treatment groups had similar baseline demographic and clinical characteristics, but Study A included older and more obese patients, a higher number of female patients, and patients with lower lung function than Study B2.
Table 1. Characteristics of study patients at baseline in the individual studies and the pooled dataset
Asthma exacerbations
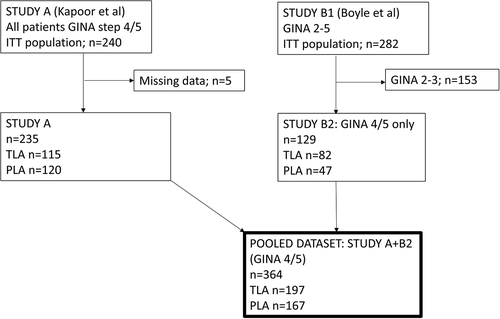
Neither Study A nor Study B2 separately showed a statistically significant difference between TLA and placebo for severe asthma exacerbations. gives a descriptive presentation of the rate of severe asthma exacerbations over 12 months for TLA and placebo in the individual studies by baseline ACQ7 (Study A, )) and ACT (Study B2, )). In both studies, the rate difference between TLA and placebo consistently increased with lower symptom control at baseline.
Figure 2. Descriptive presentation of the rate of severe asthma exacerbations over 12 months in Study A and Study B2 by baseline ACQ7 and ACT-scores for TLA-treated (solid line) and placebo-treated (dotted line) patients. (a): Baseline ACQ7 score in Study A. (b): Baseline ACT score in Study B2
presents the pooled results for Study A+ B2 by different cut-points for baseline ACQ7/ACT. A significant reduction of severe exacerbations was shown in favour of TLA over placebo across the range (59%, 51%, 48%, 41%, respectively). A cut-point of ACQ7 = 2.5 and ACT = 18 resulted in a non-significant 27% reduction (p = 0.096). A sensitivity analysis in patients aged 16 upwards was performed, as Study A contrary to Study B2 included no children. No important differences were found.
Table 2. Rate ratios for severe asthma exacerbations in the pooled dataset for different cut-off levels of baseline ACQ7/ACT scores, total AQLQ score, and AQLQ symptom domain score
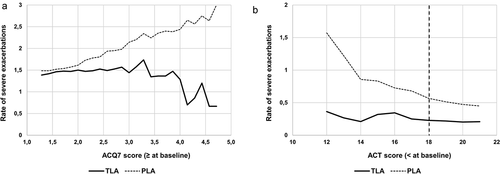
presents the pooled results for Study A+ B2 by different cut-off points for baseline AQLQ. For more symptomatic patients with severe asthma defined as AQLQ symptom domain ≤3.0, a significant 53% reduction of severe exacerbations (p = 0.011) was observed for TLA over placebo in the pooled dataset. For patients with AQLQ total ≤3.0 at baseline, the reduction of severe asthma exacerbations for TLA vs placebo was 47% (p = 0.037).
Figure 3. Rate of severe asthma exacerbations over 12 months in the pooled dataset by different cut-off points for baseline total AQLQ for TLA-treated (solid line) and placebo-treated (dotted line) patients. (a): total AQLQ score. (b): AQLQ symptom domain only
Quality of life
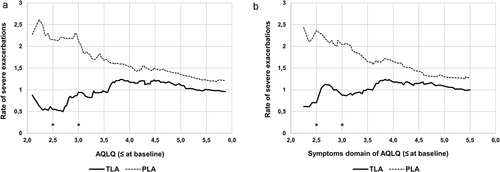
Using baseline AQLQ as a covariate, presents the proportion of responders (Minimal Clinically Important Difference (MCID) ≥0.5) [Citation9] in AQLQ after 12 months for TLA and placebo in the pooled dataset. A significant difference in the number of responders between TLA and placebo was observed for patients with baseline AQLQ ≤ 3.0 (74% vs. 43%, p = 0.04). The stopping criterium for non-significance was reached at AQLQ total = 3.5 (71% vs.55%; p = 0.11).
Figure 4. Proportion of responders in AQLQ after 12 months in the pooled dataset by different cut-off points for baseline total AQLQ for TLA (solid line) and placebo (dotted line)
Discussion
This post-hoc meta-analysis of two double-blind 12 month’s studies investigated the effect of TLA and placebo in patients with severe allergic asthma. TLA showed significant effects in more symptomatic patients on severe exacerbations (41–59% reduction) and AQLQ response (74% vs.43%) compared to placebo.
These results diverge from the full LASER trial [Citation16], which showed small and non-significant differences for severe exacerbations (8% reduction) and modest effects on AQLQ. In the 4A asthma trial [Citation9] there was an overall significant difference between TLA and placebo on AQLQ responder rate, and a non-significant effect on severe exacerbations in the sub-group of patients with symptomatic severe allergic asthma (GINA 4/5 + ACT< 18). Therefore, this meta-analysis investigating the effect of TLA on severe exacerbations was initiated, as the two studies had low power for analysis of severe exacerbations in the more symptomatic severe allergic asthma patient population and since all sub-analyses further lowered the number of patients to be included. Descriptively, there was a broad difference in severe exacerbations between TLA and placebo in more symptomatic patients – a greater effect difference as severity increased. The more symptomatic patients showed a significant reduction in severe exacerbations in favour of TLA, both for validated questionnaires (ACQ7 and ACT) and the AQLQ symptom domain.
The effect on severe exacerbation of around 50% for TLA compared to placebo in the more symptomatic patients demonstrated in this pooled analysis is of similar magnitude to the effect demonstrated by biological agents [Citation2–4]. One further possible explanation for the larger treatment response among the more severe allergic asthma patients is the association between allergy and asthma. With increased allergen load the disease becomes more severe, i.e. more difficult to control [Citation20].
Another important topic is whether patients who are sensitized to more allergens respond better to TLA than those less sensitized. The data from the two studies were not suitable for pooling. In a post-hoc analysis of the Boyle study, it has been reported that patients with ACT score <18 plus GINA 4/5 plus allergic response to 2 or more allergens had a significant reduction in the number of severe exacerbations for TLA compared to placebo (p = 0.02; n = 61). In the same subgroup, but with an allergic response to one or more allergens, the result was not significant (p = 0.07; n = 87) [Citation7]. The significance in the more sensitized group may indicate that multi-sensitization has impact on the outcome
The importance of poor control and the future risk of severe exacerbations in asthma has been demonstrated in a number of studies, using asthma control questionnaires, such as ACT and ACQ as a surrogate, quantitative estimates of control [Citation21–23]. Within these measures, the presence and severity of symptoms in predicting exacerbations are important, and they remain the second most common predictor (after a history of previous exacerbations) of future risk. The evidence for asthma control is therefore strong while questionnaires on asthma QoL focusing on symptom and non-symptom domains are less consistent in predicting future risk of exacerbation [Citation23]. It has also been shown that shorter disease questionnaires (Airways Questionnaire 20 (AQ20) and COPD Control Questionnaire (CCQ)) focusing on symptoms are more predictive than broader, more time-consuming disease questionnaires (such as AQLQ and St. George’s Respiratory Questionnaire (SGRQ)) in the prediction of exacerbations and hospitalisations [Citation24]. It is, therefore, logical to use an easily administered control questionnaire (e.g. ACQ) as an inclusion criterion in studies where severe exacerbations are the primary outcome, as used in the LASER study (ACQ7 > 1) and in the biological study programmes (ACQ6 > 1.5). In our analyses of the TLA effect on severe exacerbations, we demonstrated increasing reductions in exacerbations using cut-off points for asthma control (ACQ and ACT) as well as AQLQ (especially when using the symptoms domain of AQLQ). However, more patients were included in the pooled analyses if control questionnaires (ACQ7+ ACT) were used than if a QoL instrument was used (AQLQ total). This may indicate that validated symptom control questionnaires are better and more easily define the right patient population for predicting a positive TLA response to severe exacerbations.
Descriptively, the proportion of responders on TLA was around 70% for most baseline AQLQ scores. However, the proportion of placebo responders was low in patients with the most symptomatic severe allergic asthma (30–40%). A baseline AQLQ-value of >3.0 resulted in a placebo response rate of around 60% (see ), indicating a prominent placebo response in patients with milder symptoms, which may have lowered the possibility for TLA to show significant effects over placebo.
Allergen avoidance using air purifications could be an alternative to TLA. However, the effectiveness in asthma treatment by conventional air purification is limited and a recently published meta-analysis by Boven et al. [Citation25] showed that the only significant clinical results (AQLQ and FeNO) were demonstrated with TLA. A plausible explanation has been given by Spilak et al. [Citation26], who demonstrated that TLA significantly reduces the intrusion of airborne particles into the breathing zone with particle number concentrations being 100-fold lower compared to air-cleaners, which attempt to reduce the allergen/particle load in the whole room, rather than focused in the breathing zone.
The post-hoc nature of the analysis is a limitation in itself, and many analyses have been performed on a relatively small dataset. The analyses are exploratory rather than pre-specified in order to find possible areas for further studies. However, the selected sub-groups were outlined already in the protocol for the 4A asthma trial. In that study, it was demonstrated that more symptomatic patients (ACT<18) and/or more severe patients (GINA 4/5) showed a greater effect with TLA compared with placebo. In the meta-analysis, multiple analyses were performed, but we sequenced the analyses and stopped when no significance was reached in order to minimize multiplicity. It should also be noted that with a greater sample size for the sub-analysis, more information could have been obtained. A logical next step would be to repeat an adequately powered study to validate the sub-group findings. Other potential limitations are the differences in patient numbers, in patient populations and the use of two different baseline asthma symptom scores (ACQ and ACT) in the two studies, adding heterogeneity to the pooled dataset. As in other meta-analyses, we used a fixed factor in the model, i.e. the pooled results are approximately weighted by study size. In addition, a sensitivity analysis was performed on the 4A asthma trial, showing no difference in exacerbation rate when patients aged 15 and younger were omitted. All data, however, indicated that TLA demonstrated a better add-on effect in the more symptomatic patients, irrespective of severity based on medication load.
In conclusion, this meta-analysis of individual patient data shows a beneficial effect on severe exacerbations and quality of life for TLA over placebo in more symptomatic patients with severe allergic asthma. These outcomes support the national management recommendations for patients with symptomatic severe allergic asthma. The actual effect of TLA on severe exacerbations in the recommended patient population should be confirmed in a prospective study with larger numbers of patients.
Glossary
Acknowledgments
We thank the sponsors of the two trials Airsonett AB and Portsmouth Hospitals NHS Trust for providing data for this post-hoc analysis and Dr. Robert Boyle, Imperial College Healthcare NHS Trust, London, UK for helpful discussions.
Disclosure statement
AJC was Chief Investigator of the LASER Trial and currently conducting the LASER-48 trial funded by Airsonett. He has been on advisory boards for Sanofi Genzyme, Novartis, XIM, Glyconics and Exhalation Technology. TPB and WS have no conflicts to declare. LBJ was on the scientific advisory board and an investigator on the 4As trial. He has given paid lectures during Airsonett symposia at international conferences. GE has been on a scientific advisory board and has consulted for Airsonett and other companies. FR has no conflicts of interest to report. SP has consulted for Airsonett and other companies. JOW has been a member of scientific advisory boards and/or run clinical trials with funding from Airsonett, UCB pharma, AstraZeneca, Novartis, Allergy Therapeutics, Merck and Danone/Nutricia.
Additional information
Funding
Notes on contributors
A. J. Chauhan
Anoop J. Chauhan, MB ChB, MD, PhD, FRCP, is a Professor of Respiratory Medicine at Portsmouth Hospitals University NHS Trust and University of Portsmouth, Portsmouth, UK. His main research focus is medical technology, virus infection and severe asthma.
T. P. Brown
Thomas P. Brown, MD, is a Consultant in Respiratory Medicine at Portsmouth Hospitals NHS trust. His main research focus is lung diseases, airway obstruction and asthma management.
W. Storrar
William Storrar, MD MRCP, is a Consultant in Respiratory Medicine at Hampshire Hospitals NHS Foundation Trust, UK. His main research focus is clinical research in airways disease.
L. Bjermer
Leif Bjermer, MD, PhD, is a senior Professor in Medicine at the Department of Respiratory Medicine and Allergology, University Hospital, Lund, Sweden and a senior medical advisor at Norwegian University of Science and Technology, Trondheim, Norway. His main research focus is translational research in asthma and COPD.
G. Eriksson
Göran Eriksson, MB, PhD, is a clinical researcher at the Department of Respiratory Medicine and Allergology, University Hospital, Lund, Sweden. His main research focus is clinical research in asthma and COPD.
F. Radner
Finn Radner, PhD, is a researcher at the Department of Respiratory Medicine and Allergology, University Hospital, Lund, Sweden. His main research focus is clinical research in asthma and COPD.
S. Peterson
Stefan Peterson, PhD, is a statistician at StatMind Statistical and Mathematical Modelling, Innovation and Design AB, Lund, Sweden.
J. O. Warner
John O. Warner is Professor Emeritus Paediatrics NHLI Imperial College, London, UK and Hon. Professor University of Cape Town, South Africa. His research interests are allergic asthma, food allergy, early life origins of allergic and respiratory conditions.
References
- Global Initiative for Asthma Global Strategy for Asthma Management and Prevention. 2018. http://ginasthma.org/2018-gina-report-global-strategy-for-asthma-management-and-prevention2018
- Farne HA, Wilson A, Powell C, et al. Anti-IL5 therapies for asthma. Cochrane Database Syst Rev. 2017;9:CD010834.
- Hu J, Chen J, Ye L, et al. Anti-IgE therapy for IgE-mediated allergic diseases: from neutralizing IgE antibodies to eliminating IgE(+) B cells. Clin Transl Allergy. 2018;8(1):27.
- Lawrence MG, Steinke JW, Borish L. Cytokine-targeting biologics for allergic diseases. Ann Allergy Asthma Immunol. 2018;120(4):376–9.
- Tice JA, Campbell JD, Synnott PG, et al. The Effectiveness and Value of Biologic Therapies for the Treatment of Uncontrolled Asthma. J Manag Care Spec Pharm. 2019;25(5):510–514.
- Anderson WC III, Szefler SJ. Cost-effectiveness and comparative effectiveness of biologic therapy for asthma: to biologic or not to biologic? Ann Allergy Asthma Immunol. 2019;122(4):367–372.
- Warner JO. Use of temperature-controlled laminar airflow in the management of atopic asthma: clinical evidence and experience. Ther Adv Respir Dis. 2017;11(4):181–188.
- The National Institute for Health and Care Excellence (NICE). The Airsonett temperature-controlled laminar airflow device for persistent allergic asthma. NICE MIBs. 2014;8:2014.
- Boyle RJ, Pedroletti C, Wickman M, et al. Nocturnal temperature controlled laminar airflow for treating atopic asthma: a randomised controlled trial. Thorax. 2012;67(3):215–221. .
- Bjermer L, Eriksson G, Radner F, et al. Time to onset of improvements in quality of life from temperature-controlled Laminar Airflow (TLA) in severe allergic asthma. Respir Med. 2019;147:19–25.
- Schauer U, Bergmann KC, Gerstlauer M, et al. Improved asthma control in patients with severe, persistent allergic asthma after 12 months of nightly temperature-controlled laminar airflow: an observational study with retrospective comparisons. Eur Clin Respir J. 2015;2:2.
- Pedroletti C, Millinger E, Dahlen B, et al. Clinical effects of purified air administered to the breathing zone in allergic asthma: a double-blind randomized cross-over trial. Respir Med. 2009;103(9):1313–1319.
- Brazier P, Schauer U, Hamelmann E, et al. Economic analysis of temperature-controlled laminar airflow (TLA) for the treatment of patients with severe persistent allergic asthma. BMJ Open Respir Res. 2016;3(1):e000117.
- SMPA. Behandlingsrekommendationer för astma hos vuxna och barn. 2015. (26-43) Available from: https://lakemedelsverket.se/upload/halso-och-sjukvard/behandlingsrekommendationer/Lakemedelsbehandling-vid-astma-behandlingsrekommendation-webb.pdf
- Airsonett: Innovative Medical Technology Overview. Healthcare Improvement Scotland. 003/2015.
- Kapoor M, Storrar W, Balls L, et al. Nocturnal temperature-controlled laminar airflow device for adults with severe allergic asthma: the LASER RCT. Health Technol Assess. 2019;23(29):1–140. .
- Reddel HK, Taylor DR, Bateman ED, et al. An official American thoracic society/European respiratory society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med. 2009;180(1):59–99. .
- Van Dijk BCP, Svedsater H, Heddini A, et al. Relationship between the asthma control test (ACT) and other outcomes: a targeted literature review. BMC Pulm Med. 2020;20(1):79.
- Korn S, Both J, Jung M, et al. Prospective evaluation of current asthma control using ACQ and ACT compared with GINA criteria. Ann Allergy Asthma Immunol. 2011;107(6):474–479.
- Del Giacco SR, Bakirtas A, Bel E, et al. Allergy in severe asthma. Allergy. 2017;72(2):207–220. .
- Bateman ED, Buhl R, O’Byrne PM, et al. Development and validation of a novel risk score for asthma exacerbations: the risk score for exacerbations. J Allergy Clin Immunol. 2015;135(6):1457–64 e4. .
- Boer S, Sont JK, Loijmans RJB, et al. Development and validation of personalized prediction to estimate future risk of severe exacerbations and uncontrolled asthma in patients with asthma, using clinical parameters and early treatment response. J Allergy Clin Immunol Pract. 2019;7(1):175–82 e5.
- Loymans RJB, Debray TPA, Honkoop PJ, et al. Exacerbations in adults with asthma: a systematic review and external validation of prediction models. J Allergy Clin Immunol Pract. 2018;6(6):1942–52 e15.
- Blanco-Aparicio M, Vazquez I, Pita-Fernandez S, et al. Utility of brief questionnaires of health-related quality of life (Airways Questionnaire 20 and Clinical COPD Questionnaire) to predict exacerbations in patients with asthma and COPD. Health Qual Life Outcomes. 2013;11(1):85.
- Van Boven FE, De Jong NW, Braunstahl GJ, et al. Effectiveness of the air purification strategies for the treatment of allergic asthma: a meta-analysis. Int Arch Allergy Immunol. 2020;181(5):395–402.
- Spilak MP, Sigsgaard T, Takai H, et al. Comparison between temperature-controlled Laminar Airflow device and a room air-cleaner in reducing exposure to particles while asleep. PLoS One. 2016;11(11):e0166882.