ABSTRACT
Introduction: The organ-specific Danish cancer patient pathways (CPPs) including standard time frames were introduced in 2008-2009 securing fast tracks for cancer diagnosis and treatment. Previous studies of the CPPs have focussed on patients getting the suspected cancer diagnosis, whereas little is known about patients not getting the cancer diagnosis for which they were examined. We aimed to describe the characteristics of patients who completed a lung cancer CPP (LCPP) without getting a LC diagnosis. Furthermore, to assess the proportion of patients who had invasive procedures performed during the LCPP and radiographic examinations of the chest conducted 30 days prior to the LCPP and during the LCPP. Moreover, we aimed to describe the proportion of patients being diagnosed with any other cancer-type than LC or with non-malignant pulmonary diseases (NMPDs) during the LCPP.
Methods: The study was a retrospective population-based cohort study based on Danish national registers. Patients completing a LCPP between 1 January 2013 and 31 December 2016 without being diagnosed with LC and who were registered as initiating and completing the LCPP, a total of 35,809, were included in the study.
Results: Invasive procedures were performed in 12,986 patients (37.4%) and almost all patients had CT-scans of thorax and lungs conducted 30 days prior to or during the LCPP. During the LCPP other cancer-types than LC were diagnosed in 1,537 patients (4.3% of the study population), including other primary thoracic malignancies in 312 patients, while 6,826 patients (19.1%) were diagnosed with NMPDs, most often infections or chronic respiratory diseases of lower airways.
Conclusion: Besides diagnosing LC the LCPP may contribute significantly in diagnosing other primary and secondary cancers as well as non-malignant diseases.
Introduction
The standardized organ-specific cancer patient pathways (CPPs) were implemented in the Danish health care system from 2008 and onwards [Citation1]. The guidelines associated with the CPPs define symptoms that should lead to referral to a CPP and include standard time frames for each step from referral to diagnosis and start of initial cancer treatment. Furthermore, the most commonly employed tools for diagnostic work-up and the guideline-based treatments are stated. Finally, it is stressed when the patients must be informed about findings, diagnoses, and suggestions for treatment [Citation2]. The overarching aim of the CPPs were – together with national clinical guidelines – to improve survival of Danish cancer patients [Citation2]. Thus, the majority of Danish cancer patients should be subjected to a fast-track and harmonized diagnostic work-up and rapid onset of guideline-consistent treatment. At the time of implementation, there was no scientific evidence of the efficacy and/or cost-effectiveness of any of the CCPs [Citation1]. Lung cancer (LC) is the second most common Danish type of cancer next to breast cancer [Citation3] and the cancer type with the highest mortality [Citation4]. Since the implementation of the lung cancer CPP (LCCP), a favourable stage migration and improved survival of Danish LC patients have been observed [Citation5,Citation6] together with a diminished gap in survival compared to other Scandinavian countries [Citation7]. Following the Danish initiative, Sweden and Norway have also implemented CPPs [Citation8]. The majority of patients referred to a LCPP are not diagnosed with LC [Citation9], however the referral implies that the doctor suspected severe illness. It is unknown to what extent patients not diagnosed with LC are subjected to a perhaps stressful diagnostic work-up with invasive examinations. It is also not known if these patients are diagnosed with other types of cancers than LC during the LCPP or to which extent non-malignant pulmonary diseases (NMPDs) are diagnosed. Knowledge about patients not diagnosed with LC during LCPPs may help to improve care and planning of examinations during LCPPs and could point out which other cancers than LC and NMPDs the clinicians should be aware of during the LCPP.
The aims of the present study were therefore to describe the patients who completed a LCPP without getting a LC diagnosis and to characterise the LCPPs including invasive examinations and radiographic examinations of the chest conducted during and 30 days prior to the LCPP. Furthermore, we aimed to investigate the proportion of patients being diagnosed with another cancer than LC or NMPDs during the LCPP.
Material and methods
Study design
The study was a retrospective population-based cohort study of patients who completed a LCPP between 1 January 2013 and 31 December 2016 without being diagnosed with LC.
The Danish lung cancer patient pathway (LCPP)
The LCPP was established as a collaboration between relevant Danish clinicians and administrators in the health system. The aims of the pathway were to ensure a fast-track for diagnosis of LC and to increase the attention of the clinicians – especially general practitioners – of serious symptoms that may be indicative of LC, e.g. if a patient is > 40 years and with a history of smoking, but previously without pulmonary symptoms experiences persistent cough for 4–6 weeks, or if a similar patient with chronic bronchitis experiences a change in coughing pattern. In case of such symptoms the patient should be further investigated by radiographic imaging of the chest. If the imaging procedures support the clinical suspicion of LC, the patient should be referred to the LCPP. Furthermore, patients who are already in contact with a hospital department must be referred to a LCPP, perhaps in the same department, if clinical suspicion arises. The LCPP is terminated on the request of the patient, or if the clinical suspicion of LC is dismissed. This implies that if a non-malignant disease is suspected, the patient should, if deemed necessary, get a diagnosticwork-up outside the LCPP. The LCPP may also be terminated if a biopsy shows a pulmonary metastasis from an extra-pulmonary primary tumor.
Data sources and study population
All residents in Denmark have a unique Civil Personal Registration (CPR) number, which is used in the national Danish registers [Citation10]. The CPR number allows linkage between different registers [Citation10]. The study population was identified through the National Patient Registry (NPR) [Citation11] and the Danish Cancer Registry (DCR) [Citation12]. The NPR contains information on all patient-contacts, procedures, and diagnoses in Danish public hospitals. Private hospitals are not involved in the diagnostic work-up and treatment of LC in Denmark. The NPR is the basis for allocation of resources to the public hospitals and is therefore considered to be complete concerning resource-heavy contacts and procedures [Citation11]. The DCR is population-based and contains information on all incident cancers [Citation12].
Patients were included in the study if they were registered with either the code for referral to a LCPP (AFB26A) and/or the code for initiation of a LCPP (AFB26B) at a public hospital and registered with the code for completing the LCPP (AFB26X1) with no verified LC diagnosis in NPR and DCR. Patients who entered the LCPP but were not registered with the code for completing the LCPP within 3 months, were not included in the study. Three months were chosen as it is twice the standard timeframe from referral to start of initial treatment in a LCPP.
Outcome measures
The outcome measures were the proportion of LCPPs containing invasive clinical examinations, and the proportion of patients who had radiographic examinations of the chest conducted 30 days prior to the LCPP and during the LCPP. Moreover, the proportion of patients diagnosed with other cancers than LC including other primary cancers in the chest and the proportion of patients referred to another organ-specific CPP, either during or up to 10 days after the LCPP was completed. Ten days were chosen to allow time for obtaining a definite specific pathological diagnosis after the LCPP. Also, patients registered with NMPDs during the LCPP or within 28 days after completing the LCPP were determined. Twenty-eight days were chosen because diagnostic work-up of clinically suspected non-malignant diseases is generally performed outside the LCPP.
Invasive examinations were defined as procedures that include incision, insertion, or injection into the body. All invasive examinations that were registered more than 100 times in the NPR among the study population, during the LCPPs were included in the study. The examinations included bronchoscopies, biopsies, thoracoscopies, thoracentesis, and gastro- and colonoscopies ( and in the Appendix A for a complete list of the included invasive examinations and NPR codes for the examinations). If a patient was registered with one or more invasive examinations during the LCPP, the LCPP was categorised as being invasive. Radiographic examinations of the chest were determined as registrations of the respective codes in the NPR during the LCPP and 30 days prior to referral to the LCPP. In cases where there was more than one registration of a specific invasive or non-invasive examination per day per patient, we only included the first.
Table 1. Characteristics of the study population of patients entering and completing a Danish lung cancer patient pathway (LCPP) in the period 2013–2016 without getting a lung cancer diagnosis
Table 2. Invasive examinations, CT-scans of thorax and lungs and other radiographic examinations of the chest performed 30 days prior to LCPP and during LCPP among the study population (n = 35,809) between year 2013–2016
Table 3. Patients diagnosed with cancer (according to ICD-10 registrations in NPR) among the study population during the lung cancer patient pathway (LCPP) and within 10 days after LCPP
Diagnoses of other types of cancer than LC were determined based on registration of ICD10-codes: C00-C97 in the NPR (except ICD10-code: C34). A subsequent CPP referral was determined as a registration of the code for referral to a CPP in the NPR. NMPDs included were (with ICD-10 codes): chronic respiratory disease of the lower airways (DJ4), pneumonia (DJ1), sarcoidosis (DD86), pulmonary abscess and interstitial lung disease (DJ8), apleura effusion (DJ90-DJ919), necrotizing vasculitis (DM31), aspergillosis (DB44), lung diseases due to external agents (DJ6), and tuberculosis (DA31). The population was described by age, gender, and comorbidity at the date of entry into the study. Data on age and gender were retrieved from the Danish Civil Registration System covering information on all Danish residents and persons with temporary stay in Denmark [Citation10]. The burden of comorbidity was assessed using the Charlson’s Comorbidity Index (CCI) [Citation13] and based on registrations of diagnoses in the NPR 10 years prior to referral to the LCPP.
Statistical analysis
Analyses were conducted using SAS version 9.4. Continuous variables were described as medians with 5th and 95th percentiles and categorical variables were described as proportions (%). Statistical associations between cancer diagnoses and the LCPP containing invasive/non-invasive procedures were estimated in univariate logistic regression analyses. Statistical significance level was set at a P-value of <0.05.
STROBE guidelines
The article follows the STROBE guidelines [Citation14].
Ethical considerations
According to Danish law approval from the National Committee on Health Research Ethics was not required for this type of study as no contacts were made to patients.
Funding
The study was funded entirely by The Danish Cancer Society without external funding.
Results
Study population
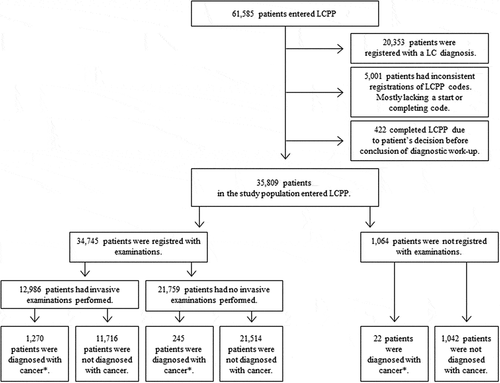
A total of 61,585 patients entered a LCPP from 1 January 2013 and completed the LCPP before 31 December 2016. LC diagnoses were registered in 20,353 patients during a LCPP, leaving 41,232 without a diagnosis of LC. Registrations of LCPP codes lacking valid dates of entering or completing the LCPP were found in 5,001 of these patients, and 422 LCPPs were discontinued due to patient’s decision before conclusion of the diagnostic work-up. When these patients were excluded, the study population consisted of 35,809 patients completing a LCPP without being diagnosed with LC ().
Patient characteristics
As seen in , the majority of the study population was between 40 and 60 years of age with a median age of 56 years. Males constituted 54.3% of the population, and 50.9% had no registered comorbidity (CCI = 0). Overall, 34,745 patients (97.0% of the study population) were registered in the NPR with clinical examinations during the LCPP, while 1,064 patients (3.0% of the study population) did not have any registrations thereof ().
Examinations performed 30 days prior to the LCPP
A total of 27,763 patients (77.5% of the study population) had either CT-scans (9,150 patients or 25.6% of the study population) or x-rays (18,613 patients or 52.0% of the study population) of the chest performed within 30 days prior to referral to LCPP (). Furthermore, it was found that 9,345 patients (26.1% of the study population) had CT-scans of abdomen made within 30 days prior to referral to LCPP (Data not shown).
Examinations performed during the LCPP
Among the study population, 24,808 patients (69.3%) had CT-scans of the thorax and lungs performed during the LCPP (). PET-scans (9,207 patients) and x-rays of thorax (8,893 patients) were conducted among 25.7% and 24.8% of the study population, respectively. CT-scans of the abdomen were performed among 15,233 patients (42.5% of the study population) (Data not shown). The most common invasive examinations performed among the study population were bronchoscopies which were performed among 11,069 patients (30.9% of the study population) ().
Among patients who were registered with clinical examinations, 37.4% had invasive examinations performed during their LCPP ().
Table 4. Univariate logistic regression analysis of the association between invasive or non-invasive lung cancer patient pathway (LCPP) and the risk of being diagnosed with cancer during LCPP and 10 days after LCPP among patients who were registered with clinical examinations during the LCPP
Cancer diagnoses and referrals to other CPPs during the LCPP
During or up to 10 days after the LCPP, 1,537 patients (1,270 patients with invasive examinations + 245 patients without invasive examinations + 22 patients with no examinations at all or in total 4.3% of the study population) were diagnosed with another cancer than LC (). The most common diagnoses were lung metastases (803 patients) and metastases in mediastinum (153 patients) (). Primary thoracic malignancies other than LC were found in 312 patients including 157 patients with cancer in lymphoid tissues, 140 patients with pleural mesotheliomas, and 15 patients with cancer in thymus (). Primary cancers of the metastases were only registered in 58 patients (5.8% of the patients registered with metastases in the lungs, pleura, and mediastinum) during or within 10 days after the LCPP. Among the 637 patients who were registered with other cancers 27% were diagnosed with either breast-, colorectal- or kidney cancer and the rest, with more rare cancer types (Data not shown). Patients diagnosed with any type of cancer during or up to 10 days after the LCPP were more likely to have gone through invasive examinations during the LCPP than patients not diagnosed with cancer (OR: 9.52 95% CI:8.29;10.93) (). Among patients not diagnosed with cancer during the LCPP, 35.3% had invasive examinations performed ().
Furthermore, 1,391 patients (3.9% of the study population) were referred to another CPP during or up to 10 days after the LCPP, primarily to CPPs for colorectal cancer and head and neck cancer ().
Table 5. Patients referred to another organ-specific cancer patient pathway (CPP) among the study population during LCPP and within 10 days after the LCPP
Non-malignant pulmonary diseases diagnosed during or after the LCPP
During the LCPP and up to 28 days after, 6,826 patients (19.1% of the study population) were diagnosed with NMPDs. The most common diagnoses being chronic respiratory disease of the lower airways in 2,536 patients and pneumonias in 2,460 patients. However, 1,033 patients were diagnosed with sarcoidosis, while pulmonary abscesses and interstitial lung diseases were found in 677 patients, pleura effusions in 650 patients, and more rare diseases in 219 patients ().
Table 6. Non-malignant pulmonary diseases (NMPD) diagnosed in 6,826 patients (according to ICD-10 registrations in NPR) among the study population during the lung cancer patient pathway (LCPP) and within 28 days after the LCPP
Discussion
This population-based study of patients who in recent years went through the Danish LCPP without being diagnosed with LC describes a group of patients that is generally ignored when assessing the outcome of and/or the quality of the diagnostic cancer work-up. However, this group of patients actually constitutes the majority of patients completing a LCPP [Citation9]. To our knowledge only one study has previously studied the outcome of organ specific Danish CPPs. In that study 7.1% of patients who entered a LCPP without being diagnosed with cancer were referred to a second CPP within 6 months of follow-up compared to 3.9% in the present study with a follow-up period of 10 days [Citation15].
Patients who enter a LCPP without being diagnosed with LC tend to be younger than patients generally diagnosed with LC since the majority of the study population was 40–60 years of age, and the majority of patients diagnosed with LC in Denmark in 2016 was 60–80 years of age [Citation16].
Almost every patient in the study population had CT-scans of thorax and lungs conducted either 30 days prior to (25.6% of the study population) or during (69.3% of the study population) the LCPP. A high number of patients had CT-scans of the abdomen performed either 30 days prior to or during the LCPP. This finding may reflect that CT-scan of the upper abdomen is a part of the diagnostic workup for suspected LC. Or it may reflect clinical suspicion of intestinal cancer prior to referral or during the LCPPs or it may be that the patients were referred to the LCPP as a result of an accidental finding on the CT-scan of abdomen or vice versa.
A group of 1,064 patients (3.0% of the study population) had no examinations registered at all in NPR during LCPP, but many of these patients had probably radiographic examinations of the chest performed prior to the LCPP.
The large proportion of patients (4.3% of the study population) being diagnosed with other types of cancers than LC during or up to 10 days after the LCPP, including other primary thoracic malignancies underscores that these patients are in a high risk of having serious diseases even though they were not diagnosed with LC during the LCPP. Only 5.8% of the patients who were registered with metastases during and 10 days after the LCPP were registered with primary cancers within the time frame. This may indicate that the studied time interval did not allow for obtaining a definite specific pathological diagnosis, which may however be reflected in the types of other organ-specific CPPs to which 3.9% of the study population were referred during or shortly after the LCPP.
As expected, there was a significant association between going through invasive examinations during the LCPP and getting a cancer diagnosis during the LCPP. Since the diagnosis of cancer is generally based on a biopsy, our finding may simply be due to confounding by indication.
The majority of patients diagnosed with other cancers than LC during the LCPP had lung metastases. The most frequent primary cancer diagnosed during the LCPPs was cancer in lymphoid tissues, probably due to the predominance for an intra-thoracic onset and involvement [Citation17,Citation18]. The prevalence of other cancer diagnoses than LC and the demands for gastroscopies, colonoscopies, and other examinations stress the need of a close collaboration between relevant specialities in the diagnostic process of cancer. This may in particular be relevant during the LCPP as metastases are frequent in the lungs.
A large group of patients 6,826 (19.1% of the study population) were diagnosed with NMPDs during or 28 days after the LCPP. Many of these diagnoses represent diseases which may mimic LC and have important consequences regarding treatment. This finding reflects that these patients besides from having the diagnosis of LC dismissed actually benefit from participating in the LCPP, even though diagnostic work-up probably was performed after completion of the LCPP, and some of the diagnoses, especially the chronic respiratory diseases of lower airways, may have been known before the LCPP.
Strengths and limitations
This study presents data from a large national population-based study, on a relevant, but rather undescribed group of patients that have been referred to the Danish LCPP, and who were registered without a diagnosis of LC. The large study population has enabled us to present an overview of the outcome of this diverse group of patients and provide multivariate, in-depth analyses and results.
Limitations do apply. We have only been able to accurately register the diagnoses of other types of cancers, referral to other CPPs, and NMPDs in relation to the LCPP and shortly after. Some patients may have been diagnosed later, and thus we may have underestimated the true diagnostic yield of the LCPP in this group of patients. Supplementing our data with data from other sources e.g. medical records or prospectively collected data could have provided validation and additional information.
Conclusion
This large population-based study based on 35,809 patients contributes with knowledge about a population, which is inadequately described in the literature: patients completing a LCPP without being diagnosed with LC. A large proportion of patients were exposed to uncomfortable and to some extent risky invasive procedures, and 1,537 patients were diagnosed with another type of cancer than LC during the LCPP, including 312 patients with other primary thoracic malignancies. This underlines that LCPP besides diagnosing LC may contribute significantly in diagnosing other primary and secondary cancers in lung, pleura and mediastinum as well as clinically important non-malignant diseases.
Disclosure of potential conflicts of interest
No potential conflict of interest was reported by the author(s).
Additional information
Notes on contributors
Therkildsen Ditte Skadhede
Therkildsen Ditte Skadhede is Master Sci. in Public Health and is working as a Senior consultant within the area of health economics. She has previously worked at the Danish Cancer Society within the field of cancer epidemiology.
Christensen Jane
Jane Christensen is Master Sci. in statistics and is statistical consultant within the field of cancer epidemiology at the Danish Cancer Society.
Andersen Ole
Ole Andersen is M.D, specialist in Pediatrics, Doctor Med. Sci., and Master of Health Management. He has previously been consultant and head of department within Pediatrics and consultant at the Danish Health Authority, and adviser at the Norwegian Health Authority, in the Danish Heath Region Zealand, and at the Danish Cancer Society. He is now retired.
Thomsen Linda Aagaard
Linda Aagaard Thomsen is PhD Pharm and head of the Science to Society group at the Danish Cancer Society Research Center focusing on bridging the gap between research and implementation in clinical practice with the aim of improving the clinical, organizational, and patient experienced quality of cancer care. She is an experienced health services researcher with core competences within pharmacoepidemiology, health economics, implementation science, and quality development in health care. She has been PI on a number of studies aimed at development or implementation of complex health care interventions in primary or secondary health care. She is member of the steering committee of the European Cancer League’s Access to Medicines Task Force, member of the program board of the International Cancer Benchmarking Partnership, member of the scientific board for the Danish implementation of the WHO “Medication without harm” initiative, and scientific advisor for the patient representatives of the Danish Medicines Council.
Rasmussen Torben Riis
Torben Riis Rasmussen is M.D, PhD, and clinical associated professor. He is Senior consultant in Pulmonary Medicine at Aarhus University Hospital. He has previously worked as Senior consultant and chairman at Danish Lung Cancer Group.
Christensen Niels Lyhne
Niels Lyhne Christensen is M.D, PhD and clinical associate professor. He is medical doctor at the Department of Pulmonary Medicine and Allergy Aarhus University Hospital. He has previously worked as PhD-student and quest researcher at the Danish Cancer Society.
References
- Probst HB, Hussain ZB, Andersen O. Cancer patient pathways in Denmark as a joint effort between bureaucrats, health professionals and politicians-A national Danish project. Health Policy (New York). 2012;105: 65–10.
- Danish Health Authority. Pakkeforløb for lungekræft 2012 [Lung cancer patient pathway 2012] (In Danish) [Internet]. 2012. Available from: https://lungekraeft.com/wp-content/uploads/2016/11/pakkeforloeb-for-lungekraeft-sundhedsstyrrelsen.pdf.
- The Danish Health Data Authority. Nye kræfttilfælde i Danmark [Cancer incidenses in Denmark] (In Danish) [Internet]. 2019. Available from: https://sundhedsdatastyrelsen.dk/da/tal-og-analyser/analyser-og-rapporter/sygdomme/kraeft-_-cancerregisteret
- The Danish Health Data Authority. Dødsårsagsregisteret 2016 [Cause of death register 2016] (In Danish) [Internet]. 2017. Available from: https://sundhedsdatastyrelsen.dk/da/tal-og-analyser/analyser-og-rapporter/andre-analyser-og-rapporter/doedsaarsagsregisteret
- Danish Lung Cancer Group & Danish Lung Cancer Register. Årsrapport 2009 [Annual report 2009] (In Danish) [Internet]. 2010. Available from: https://www.lungecancer.dk/rapporter/aarsrapporter/
- Danish Lung Cancer Group & Danish Lung Cancer Register. Årsrapport 2017 [Annual report 2017] (In Danish) [Internet]. 2017. Available from: https://www.lungecancer.dk/rapporter/aarsrapporter/
- Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391:1023–1075.
- Christensen NL, Jekunen A, Heinonen S, et al. Lung cancer guidelines in Sweden, Denmark, Norway and Finland: a comparison. Acta Oncol (Madr). 2017;56:943–948.
- The Danish Health Data Authority. Årsopgørelse 2016 - Monitorering af kræftområdet [Annual report 2016 -Monitoring cancer] (In Danish) [Internet]. 2017. Available from: https://www.esundhed.dk/Emner/Kraeft/Pakkeforloeb
- Pedersen CB. The Danish civil registration system. Scand J Public Health. 2011;39:22–25.
- Lynge E, Sandegaard JL, Rebolj M. The Danish national patient register. Scand J Public Health. 2011;39:30–33.
- Gjerstorff ML. The Danish cancer registry. Scand J Public Health. 2011;39:42–45.
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383.
- STROBE Statement [Internet]. Univ. Bern. 2009 [cited 2021 Mar 25]. Available from: https://www.strobe-statement.org/index.php?id=available-checklists
- Nielsen N, Vedsted P, Jensen H. Risk of cancer and repeated urgent referral after negative investigation for cancer. Fam Pract. 2018;35: 582–588.
- Danish Lung Cancer Group & Danish Lung Cancer Register. Årsrapport 2016 [Annual report 2016] (In Danish) [Internet]. 2017. Available from: https://www.lungecancer.dk/rapporter/aarsrapporter/
- Mack TM. Sarcomas and other malignancies of soft tissue, retroperitoneum, peritoneum, pleura, heart, mediastinum, and spleen. Cancer. 1995;75:211–244.
- Jones SE, Fuks Z, Bull M, et al. Non-hodgkin’s lymphomas iv. clinicopathologic correlation in 405 cases. Cancer. 1973;31:806–823.
Appendix A.
Table A1. Invasive examinations performed among the study population (n = 35,809) 30 days prior to the lung cancer patient pathway (LCPP) and during the LCPP between year 2013–2016