ABSTRACT
Eosinophils have a broad range of functions, both homeostatic and pathological, mediated through an array of cell surface receptors and specific secretory granules that promote interactions with their microenvironment. Eosinophil development, differentiation, activation, survival and recruitment are closely regulated by a number of type 2 cytokines, including interleukin (IL)-5, the key driver of eosinophilopoiesis. Evidence shows that type 2 inflammation, driven mainly by interleukin (IL)-4, IL-5 and IL-13, plays an important role in the pathophysiology of eosinophilic airway diseases, including asthma, chronic rhinosinusitis with nasal polyps, eosinophilic granulomatosis with polyangiitis and hypereosinophilic syndrome. Several biologic therapies have been developed to suppress type 2 inflammation, namely mepolizumab, reslizumab, benralizumab, dupilumab, omalizumab and tezepelumab. While these therapies have been associated with clinical benefits in a range of eosinophilic diseases, their development has highlighted several challenges and directions for future research. These include the need for further information on disease progression and identification of treatable traits, including clinical characteristics or biomarkers that will improve the prediction of treatment response. The Nordic countries have a long tradition of collaboration using patient registries and Nordic asthma registries provide unique opportunities to address these research questions. One example of such a registry is the NORdic Dataset for aSThmA Research (NORDSTAR), a longitudinal population-based dataset containing all 3.3 million individuals with asthma from four Nordic countries (Denmark, Finland, Norway and Sweden). Large-scale, real-world registry data such as those from Nordic countries may provide important information regarding the progression of eosinophilic asthma, in addition to clinical characteristics or biomarkers that could allow targeted treatment and ensure optimal patient outcomes.
Introduction
Eosinophilic airway diseases encompass a broadly heterogeneous group of pathologies characterised by elevated levels of eosinophils in the blood and/or tissues[Citation1]. Despite great improvements in our understanding of eosinophils, there are still many challenges in evaluating and treating eosinophilic diseases in clinical practice. Moreover, there is a distinct need for real-world information on disease progression and identification of treatable traits that will aid the prediction of treatment response. The Nordic countries have a long tradition of collaboration using patient registries[Citation2], which may provide unique opportunities to address important research questions. This review article summarises the available evidence on eosinophil biology and pathophysiology of eosinophilic airway diseases, with a focus on Nordic research to further our understanding of eosinophilic airway diseases.
Basic science of eosinophils
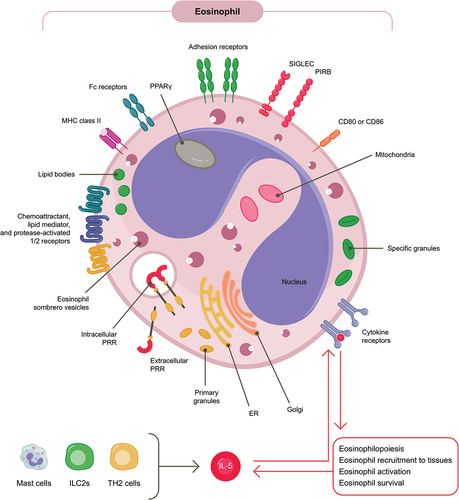
Eosinophils are involved in a variety of regulatory and homeostatic functions [Citation3,Citation4]. Through their vast array of cell surface receptors and large specific secretory granules that store proteins including ribonucleases, cytokines, enzymes and growth factors, eosinophils are well equipped for extensive interactions with their environment ()[Citation4]. Eosinophils usually develop and differentiate from pluripotent progenitors in the bone marrow, before they migrate in the blood to various tissues (for homeostatic purposes) or are recruited to sites of type 2 inflammation [Citation3–5]. However, human studies suggest that in patients with severe asthma, eosinophil progenitors also migrate to the airways where they undergo differentiation in situ[Citation6]. Their development and differentiation in the bone marrow is tightly regulated by transcription factors, as well as interleukin (IL)-5, IL-3 and granulocyte-macrophage colony-stimulating factor (GM-CSF)[Citation4]. The activation, survival and recruitment of eosinophils under normal physiological conditions is largely driven by IL-5, a key cytokine in eosinophil regulation that is produced by T helper type 2 cells (TH2), eosinophil progenitors, mast cells and type 2 innate lymphoid cells (ILC2s) (). IL-5 production is in part driven by epithelium-derivate alarmins, including IL-33, IL-25 and thymic stromal lymphopoietin (TSLP) [Citation7–10]. In turn, eosinophils exert multiple effects on other cells by releasing mediators that activate cell surface receptor signalling[Citation11]. Under homeostatic conditions, eosinophils modulate adaptive immune responses and play a role in tissue repair and restorative tissue remodelling; at sites of inflammation, they release inflammatory mediators that contribute to the innate immune response and disease pathogenesis, including excessive tissue remodelling [Citation3–5,Citation12].
Figure 1. Eosinophils are equipped with an array of cell surface receptors and intracellular mediators that facilitate interactions with their microenvironment.
Figure 1. ER, endoplasmic reticulum; IL, interleukin; ILC2s, type 2 innate lymphoid cells; MHC, major histocompatibility complex; PAR, protease-activated receptor; PIRB, paired immunoglobulin-like receptor B; PPAR, peroxisome proliferator-activated receptor; PRR, pattern-recognition receptor; SIGLEC, sialic acid binding immunoglobulin-like lectin; TH2, T helper type 2.
In murine studies, evidence suggests that different eosinophil subpopulations exist, including resident eosinophils in various tissues and inducible eosinophils [Citation11,Citation13–15]. In mice, lung resident and inducible subpopulations display differing morphological and phenotypical features; however, the presence of regulatory and inducible eosinophils is still to be conclusively shown in humans, with only a discrimination between tissue and peripheral blood eosinophils demonstrated thus far[Citation16]. Progress in basic eosinophil research has been somewhat hampered by technical challenges such as the large number of intrinsic RNases in eosinophils, the easily triggered degranulation of human eosinophils, and difficulties in obtaining sufficient numbers of purified cells from blood or inflamed tissue to perform mediator assays, single-cell RNA sequencing or long-term cell culture [Citation11,Citation13,Citation16]. Nonetheless, it is evident that different biological contexts are potentially associated with different activation profiles of eosinophils that permit unique biological functions [Citation13–15,Citation17]. For example, eosinophils from patients with severe non-allergic eosinophilic asthma have higher expression of IL-3, IL-5 and GM-CSF receptor α-chains than those from patients with non-severe allergic asthma[Citation18]. This indicates that different endotypes are likely to have different eosinophil activation and recruitment drivers. Furthermore, in patients with allergic disease or asthma, significantly more circulating eosinophils are hypodense compared with healthy patients [Citation19,Citation20], and hypodense eosinophils have increased expression of cell surface receptors CD122, CD69 and CD4[Citation21]. Characterisation of eosinophil subpopulations is therefore an important aim of eosinophil research and will shed further light on the many roles they play in health and disease.
Eosinophils in health and in disease progression
Eosinophils in immunity and health
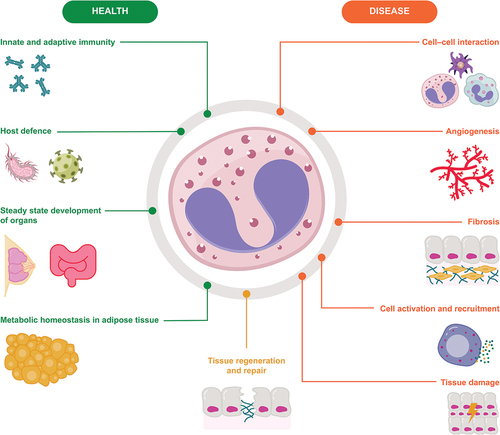
Eosinophils are present in a range of tissues in healthy individuals, including the thymus, adipose tissue, gastrointestinal tract, mammary glands, uterus and lungs [Citation11,Citation22]. Their constitutive presence suggests that they contribute to normal biological processes and there is growing evidence for their involvement in maintaining homeostasis and human health (). In multiple target tissues, eosinophils actively regulate innate and adaptive immune functions. As effector cells in innate immunity, they play an important role in host defence against microbial pathogens including fungi, bacteria and viruses, and underlie associated tissue inflammation[Citation3]. In vitro studies (using human and mouse eosinophils) and in vivo studies (using a mouse model of eosinophilia), show that during bacterial infection, eosinophils release mitochondrial DNA, which along with granule proteins, forms extracellular structures that bind to and eliminate bacteria[Citation23]. Further, in vitro studies (using human blood eosinophils and human-derived eosinophil cell lines and proteins) suggest that protection against viruses is mediated through eosinophil nitric oxide production and the release of eosinophil-derived granule proteins including eosinophil cationic protein, eosinophil-derived neurotoxin and Charcot-Leyden crystal protein/galectin-10 [Citation24–27]. Murine and human data also suggest that eosinophils interact with various immune cells including T lymphocytes and dendritic cells, and can act as antigen-presenting cells during allergen challenge or helminth infection [Citation3,Citation28,Citation29]. More recently, data from patients with several different neoplasms have shown that eosinophils may play a role in anti-tumour immunity; however, they are thought to be a source of both anti-tumorigenic and pro-tumorigenic molecules and their exact role is, as yet, unclear[Citation30].
Although data on the functions of human gastrointestinal eosinophils are lacking, mouse models show that gut-residing eosinophils are involved in the maintenance of the protective mucosal barrier and interact with other immune cells that provide immunity to pathogens present in the gut lumen[Citation31]. Murine data, supplemented by human data, show that eosinophils play various roles in adipose tissue, including sustaining adipose tissue homeostasis to promote healthy ageing, maintenance of alternatively activated macrophages (which play a crucial role in glucose homeostasis) and the development of beige fat (which improves glucose tolerance, insulin reactivity and protects against obesity) [Citation32–34]. The increase in eosinophils evident in human abdominal aortic aneurysm disease has been shown to be regulatory in mice, playing a protective role through macrophage and monocyte polarisation[Citation35]. Finally, in murine lung tissue, a subset of homeostatic eosinophils participates in the fine-tuning of respiratory immune responses by preventing type 2 inflammatory responses to low doses of allergens[Citation13]. Together, this growing body of data highlights a varied range of roles for eosinophils in healthy tissue.
Eosinophils in airway diseases
It is well understood that pathological type 2 inflammation occurs due to dysregulation of innate and adaptive immune responses[Citation36]. The role of eosinophils in mediating this inflammation is well established [Citation9,Citation10] and type 2 inflammation is considered central to the pathophysiology of eosinophilic diseases such as eosinophilic asthma, chronic rhinosinusitis with nasal polyps (CRSwNP) and eosinophilic granulomatosis with polyangiitis (EGPA) [Citation37–40]. In these diseases, a cycle of chronic inflammation is created at the pathological site, whereby the inflammatory response is amplified as the eosinophils recruited to the site secrete mediators leading to tissue damage[Citation15]. While the number of eosinophils at such sites is regulated by IL-5-induced production and recruitment to tissue by chemokines, data also suggest that eosinophil apoptosis is delayed in patients with asthma, thus prolonging tissue residency and aggravating eosinophilic inflammation[Citation41]. Conversely, corticosteroids induce apoptosis in human eosinophils at clinically relevant concentrations, likely contributing to their anti-inflammatory effect in eosinophilic asthma [Citation41–43]. Stromal interactions are considered relevant to prolonged tissue-resident eosinophil survival, which may be mediated by the cytokines IL-3 or GM-CSF [Citation44] (additional to any local tissue IL-5 generation) and potentially other factors including tumour necrosis factor (TNF)-α[Citation45] and polyamine spermidine[Citation46]. The signalling pathways that facilitate eosinophil-induced inflammation are a well-established therapeutic target for eosinophilic diseases.
Several biologic therapies have been developed to target type 2 inflammatory factors [Citation47–52]. Mepolizumab and reslizumab are monoclonal antibodies that target IL-5. Benralizumab targets the IL-5 receptor. Dupilumab and omalizumab target the IL-4/13 receptor and immunoglobulin (Ig)E, respectively, while tezepelumab blocks TSLP. All of these biologics have been shown to suppress aspects of type 2 inflammation, and additional to the IL-5-directed therapies, a reduction in the number of eosinophils in airway tissue has also been shown with omalizumab and tezepelumab [Citation53,Citation54]. Anti-IL-5 therapies have been associated with clinical benefits in several eosinophilic diseases. However, not all eosinophil populations are entirely dependent upon IL-5; cluster analyses of sputum sample gene expression identify distinct asthma groupings containing eosinophils, suggesting that there may be different underlying pathophysiology with elevated airway eosinophils in asthma[Citation55]. This highlights a need for further predictive biomarkers that reflect these different processes, and work is underway to determine the characteristics and possible significance of eosinophil populations in blood and tissue under different circumstances. Given the complex functionality of eosinophils, consideration of both their homeostatic and detrimental effects is needed in the development of anti-eosinophilic treatments. As such, it is important to consider the so-called normal range of eosinophil counts in healthy individuals, and whether a minimum number of eosinophils is needed to maintain healthy physiological functions[Citation39]. Below, we summarise the successes and challenges of treating eosinophilic diseases to date, with particular reference to Nordic countries and the promise of their broad range of patient registries.
The role of eosinophils in different airway diseases
Asthma
Asthma is a major health risk in developed or high-income countries [Citation56]; Nordic countries are no exception to this, with asthma remaining a risk factor for mortality [Citation57,Citation58]. Patients with severe asthma have poor symptom control despite high-dose treatment with corticosteroids and additional controller therapy, and represent a major clinical challenge[Citation59]. A large proportion of patients with difficult-to-control asthma also have at least one comorbidity [Citation60,Citation61], and these have a great impact on healthcare costs and health-related quality of life (HRQoL). Novel biologic treatments may benefit patients with severe asthma, but they must be targeted appropriately[Citation59].
Asthma is a complex, heterogeneous disease with different inflammatory endotypes[Citation62]. Type 2 inflammation plays a central role in allergic and non-allergic eosinophilic asthma and in mixed granulocytic asthma[Citation63]. The contribution of eosinophils to airway inflammation is well recognised, with higher blood eosinophil counts predicting future exacerbation risk and poor asthma control in patients[Citation64]. For example, in a Swedish registry study, a higher blood eosinophil count was associated with more frequent asthma exacerbations[Citation65]. In a Finnish study, higher (≥300 cells/µL) blood eosinophil counts were associated with more outpatient visits and higher total- and asthma-related healthcare resource utilisation costs than lower counts (<300 cells/µL)[Citation66]. As such, the cytokines involved in type 2 inflammatory pathways are important treatment targets. Of the current therapies targeting type 2 inflammation, benralizumab, dupilumab, mepolizumab, omalizumab and reslizumab are approved for the treatment of severe asthma [Citation47–49,Citation51]. Tezepelumab was recently approved by the U.S. Food and Drug Administration, but awaits European Medicines Agency approval. In the PATHWAY and the NAVIGATOR trials performed in patients with severe asthma, tezepelumab-treated patients had reduced exacerbation rates and showed improvements in lung function, HRQoL and asthma control[Citation52].
Mepolizumab treatment is associated with clinically relevant reductions in exacerbation rates in patients with severe eosinophilic asthma and a history of exacerbations [Citation67,Citation68], and modelling has identified baseline blood eosinophil count as the key biomarker for predicting treatment response in these patients[Citation69]. Mepolizumab primarily targets systemic inflammation, through inhibition of IL-5 signalling to the bone marrow, reducing mature eosinophilic development, whilst leaving unaffected immature eosinophil progenitor populations which can circulate to tissue sites. Consistent with this, localised eosinophilopoiesis has been demonstrated in the airways (i.e. in situ) in severe eosinophilic asthma and there is a lesser, albeit noticeable, reduction in sputum eosinophils in comparison with the impact on blood eosinophils[Citation70]. The nature of the eosinophil populations that remain within the airways is undefined, but as their presence is not associated with an increased risk of severe disease exacerbation[Citation71], their recruitment may be part of a regulatory innate immune response. This is an area with a need for deeper understanding.
Mepolizumab has also been shown to improve HRQoL [Citation72] and to have an oral corticosteroid (OCS)-sparing effect in patients with severe eosinophilic asthma[Citation73]. Clinical benefits (including reduced exacerbations, improved lung function and HRQoL) in addition to an OCS-sparing effect have also been shown in studies of reslizumab and benralizumab in patient populations similar to those included in the mepolizumab trials [Citation74–80]. Furthermore, the relationship between baseline blood eosinophil counts ≥150 cells/µL (0.15 x 109/L) and response to treatment was confirmed in post hoc analyses of the benralizumab trials[Citation81]. Similarly, patients with moderate-to-severe uncontrolled asthma who received dupilumab treatment in a Phase III trial had significantly lower rates of severe asthma exacerbations, better lung function and better asthma control than those who received placebo, with greater benefits seen in those with higher baseline blood eosinophil counts[Citation82]. Finally, omalizumab has been shown to significantly reduce the rate of asthma exacerbations, severe exacerbations and emergency visits in patients who had inadequately controlled severe persistent allergic asthma, despite high-dose ICS and long-acting β2-agonist (LABA) therapy[Citation83]. Together these large, Phase III clinical studies have demonstrated clinical benefits in patients with severe asthma when targeting type 2 inflammation.
Blood eosinophil count is the best available biomarker for predicting which patients with severe eosinophilic asthma are most likely to respond to anti-IL-5 biologics, with higher blood eosinophil counts related to greater treatment response[Citation84]. Future research should evaluate whether additional biomarkers can supplement blood eosinophil count and improve the predictive selection of populations most responsive to biologic therapy.
Recent data from a large cohort of patients with severe asthma from U-BIOPRED, a European multicentre study, supports the use of urinary leukotriene E4 (LTE4) and prostaglandin D2 (PGD2) metabolites as potential non-invasive biomarkers to guide molecular phenotyping of asthma, in addition to the selection of biologics that target type 2 inflammation[Citation85]. This study included the largest evaluation of urinary eicosanoid metabolites in individuals with and without asthma to date, and showed that LTE4 levels were significantly higher in all asthma groups compared with healthy participants[Citation85]. PGD2 metabolites were also elevated in patients with asthma compared with healthy participants[Citation85]. Moreover, there was a strong association between both urinary LTE4 and PGD2 metabolites and clinical markers of eosinophilic type 2 inflammation, such as FeNO, blood and sputum eosinophils, serum periostin and IL-13. The associations were first observed at the baseline visit, (n = 597) and reproduced in a longitudinal follow-up visit (n = 302). In the same study, the strong relationships between LTE4 and PGD2-metabolites and markers of type 2 inflammation were also validated externally using data from adolescents with severe or controlled persistent asthma[Citation85]. Data from a Swedish real-world biocohort study (BIOCROSS) showed that the expected reduction in blood eosinophils during anti-IL-5 treatment was associated with decreased concentrations of urinary LTE4[Citation86], further supporting the role of LTE4 as a marker of type 2 inflammation. The measurement of urinary eicosanoids in these studies was performed by mass spectrometry [Citation85,Citation86], but urinary LTE4 and the main metabolites of PGD2 can also be measured by established immunoassays [Citation87] that are simpler to apply in clinical laboratories.
Chronic rhinosinusitis with nasal polyps
CRSwNP is a clinical challenge. It is a chronic disease, with symptoms including loss of sense of smell and chronic nasal obstruction that can negatively impact sleep and HRQoL [Citation40,Citation88–90]. Although the aetiology of CRSwNP involves both eosinophilic and neutrophilic pathways, it is clear that eosinophils play an important role in some endotypes, particularly in the recurrence of NP among patients who are refractory to standard treatments [Citation40,Citation91–93]. Eosinophilic asthma and CRSwNP frequently co-exist, and this combination is associated with greater disease burden and worse outcomes compared with either severe asthma or CRSwNP alone [Citation94–96]. Data on whether asthma is a comorbidity of CRSwNP, or vice versa, are currently limited.
Treatment options for CRSwNP include nasal steroids, nasal douching, OCS and surgery (to reduce the NP tissue and improve access for local medical treatment)[Citation40]. Dupilumab, omalizumab and mepolizumab are approved for the treatment of CRSwNP [Citation48–50,Citation97] following Phase III trials in which the occurrence of sinonasal surgery and corticosteroid use were reduced and symptoms were improved in patients with CRSwNP [Citation96–98]. Benralizumab is currently under investigation for use in CRSwNP[Citation99].
Importantly, the 2020 European position paper on rhinosinusitis and NP states that the indication for biological treatment in CRSwNP should be the presence of bilateral NP following functional endoscopic sinus surgery as well as three of the following criteria: evidence of type 2 inflammation, need for or contraindication to systemic corticosteroids, significantly impaired quality of life, significant loss of smell and diagnosis of comorbid asthma[Citation40]. Biologic treatment should be evaluated after 16 weeks and 1 year based on reductions in NP size and need for systemic corticosteroids, as well as improvements in HRQoL and sense of smell and reductions in the impact of comorbidities[Citation40]. A need for phenotyping and endotyping patients with CRSwNP to direct treatment appropriately was also identified[Citation40]. While the data and guidelines for biologic therapies in CRSwNP provide a good basis for improving treatment options, more studies are still needed to fully elucidate their long-term clinical impact.
Chronic idiopathic eosinophilic pneumonia
Idiopathic chronic eosinophilic pneumonia (ICEP) is a rare disease characterised by systemic and localised lung eosinophilia with symptoms including dyspnoea, cough and general symptoms such as arthralgia, fever, weight loss and hypoxemia of varying severity [Citation100,Citation101]. Bronchoalveolar lavage (BAL) fluid in CEP contains a broad range of cytokines associated with local type 2 inflammation, although the type 1 cytokines IL-2 and IL-12 are also present [Citation102–104]. Furthermore, there is evidence that in CEP, BAL fluid eosinophils are activated, have locally augmented secretion of their cytotoxic, cationic granule-stored contents, decreased apoptosis and longer survival than their counterparts in the peripheral blood. The standard treatment for ICEP is OCS; however, the relapse rate with OCS tapering is high and long-term OCS use can have a deleterious effect [Citation100,Citation105,Citation106], highlighting a need for alternative therapies. Open-label treatment with mepolizumab has been shown to reduce relapses, blood eosinophil counts, OCS use and lung infiltrates in patients with relapsing ICEP[Citation107]. Future large-scale randomised controlled trials evaluating anti-IL-5 therapies in ICEP are therefore warranted.
Eosinophilic granulomatosis with polyangiitis
EGPA is a multi-system, necrotising vasculitis affecting the small-to-medium blood vessels in various organs, and is typically preceded by a prodromic phase with increasingly severe asthma and eosinophilia [Citation108–112]. EGPA is a heterogeneous disease; the frequency of organ involvement differs between patients with anti-neutrophil cytoplasmic antibody (ANCA)+ and ANCA- disease, as does response to treatment [Citation109,Citation112–114]. Several biomarkers for EGPA disease activity have been suggested, including eosinophil counts and IgE levels, which differentially affect the risk of disease relapse[Citation115].
Currently, mepolizumab is the only eosinophil-targeting biologic approved for the treatment of EGPA[Citation49]. Recently, benralizumab and reslizumab have shown efficacy in the treatment of EGPA in a number of case studies and a pilot study, respectively [Citation116–122]. In the pilot study, responders to reslizumab experienced reduced OCS use and no exacerbations[Citation116]. While studies conducted to date confirm that eosinophils are central to EGPA disease pathology, further analyses are needed to identify appropriate clinical biomarkers to separate EGPA phenotypes and direct treatment appropriately.
Hypereosinophilic syndrome (HES)
Hypereosinophilia is generally defined as a persistent blood eosinophil count of ≥1500 cells/µL (1.5 x 109/L); when this is accompanied by organ damage and is not secondary to other causes of eosinophilia, a diagnosis of HES may be made[Citation1]. HES is a rare and heterogeneous condition with multiple different classifications [Citation1,Citation123–128]. Patients present with a plethora of symptoms, including dermatologic, pulmonary, gastrointestinal, neurological and cardiac manifestations [Citation129,Citation130], with cardiovascular complications shown to be associated with poor prognosis[Citation1]. As there are no validated biomarkers for HES, an elevated blood eosinophil count is often considered to be a surrogate marker for tissue eosinophilia and organ damage.
The cornerstone of treatment for HES has traditionally been corticosteroids and immunosuppressive therapies; however, neither treatment option provides clinical benefits in all patients with HES and both can cause long-term complications, highlighting a need for better therapies[Citation131]. Other therapeutic options include the tyrosine kinase inhibitor imatinib in those patients with the tyrosine kinase fusion (FIP1L1-PDGFRA-positive), and eosinophil-targeting therapies [Citation127,Citation132]. Mepolizumab is currently the only eosinophil-targeting biologic approved for the treatment of HES [Citation49]; with benralizumab currently under investigation in this patient population[Citation133]. A Phase III study in patients with FIP1L1-PDGFRA–negative HES demonstrated reductions in blood eosinophil count and a reduced number of flares over 32 weeks with subcutaneous mepolizumab 300 mg versus placebo[Citation134]. Although the available data to date clearly show that IL-5 contributes to elevated eosinophil counts in many patients, for some patients, anti-IL-5 treatment does not affect hypereosinophilia[Citation134], indicating causes independent of IL-5. These findings indicate a need to exclude known causes of hypereosinophilia before a diagnosis of HES is reached, and that further research is needed into biomarkers that predict response to HES treatment and the most appropriate way to target treatment.
Furthering our knowledge of eosinophilic diseases using data from registries
Through the development of anti-eosinophilic treatments across the eosinophilic diseases described above, a number of challenges and directions for future research have been identified. These include: the need to identify events that may predict development of or progression to severe disease and any opportunities for early disease modification; the need to identify treatable traits, including clinical characteristics or biomarkers that will further improve the predicted response to treatment; and the need to understand comorbidities and their impact on prognosis. The ongoing requirement for real-world evidence of the long-term efficacy, safety and economic impact of biologic therapies is also clear. Moreover, it will be important to further our understanding of the most appropriate timing to introduce biologic treatments in any given patient, as well as how long to continue with treatments and whether switching or a combination of biologics may be appropriate treatment strategies in some patients. The Nordic countries have a long tradition of collaboration using patient registries, and Nordic asthma registries provide a unique opportunity to address some of these questions.
The promise of population-based registries
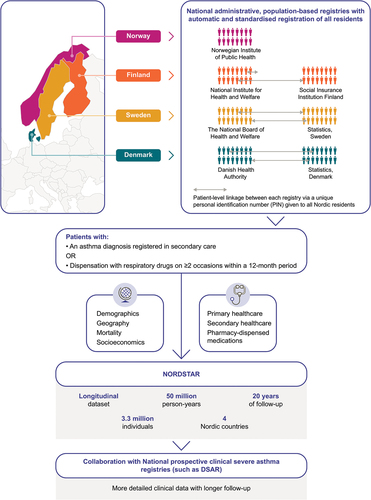
All five Nordic countries (Denmark, Finland, Iceland, Norway and Sweden) have nationwide registries that contain virtually all individuals residing in those countries[Citation2]. These registries have similar data structure and validity and use personal identity numbers, thus enabling linking of medical records between multiple registries and clinical studies to perform large cohort studies. Such studies have long follow-up periods and provide a broad wealth of clinical data, with increased statistical power versus smaller studies[Citation2]. In the case of eosinophilic diseases, large-scale population-level data with long follow-up periods and without loss to follow-up would enable assessment of disease trajectories and may identify windows of opportunity for earlier interventions.
The potential of the Nordic registry system has been realised in asthma with the development of the NORdic Dataset for aSThmA Research (NORDSTAR). NORDSTAR is an asthma research consortium with a longitudinal dataset comprising all 3.3 million individuals with asthma from four Nordic countries, with a total of approximately 50 million person-years of follow up (). The consortium is governed by the Nordic Severe Asthma Network, consisting of Nordic severe asthma specialists, and aims to improve the lives of patients with severe asthma through research and education[Citation132]. NORDSTAR is the largest asthma database globally and includes patient-level data on demographics and socioeconomics, mortality, primary and secondary healthcare resource utilisation and costs, and medication dispensing[Citation133]. It is anticipated that NORDSTAR will be updated to also include data on blood eosinophil counts and IgE levels from national laboratory databases. Within each country, patient-level linkage between registries has been conducted, enabling the extraction and amalgamation of patient-level data across all relevant data sources[Citation133]. The key research aim for NORDSTAR is to describe the trajectory of asthma from mild through to severe disease and remission, identifying any risk factors that may be associated with the development of these disease stages. This unique approach will enable longitudinal follow-up of patients with asthma in contemporary, routine clinical care, with many aspects of NORDSTAR-based research generalisable to other settings globally.
Current Nordic asthma registries
In addition to the population-based registries described above, there are several asthma-specific registries in Nordic countries that have the benefit of similar data structure and validity. The Danish Severe Asthma Register (DSAR) is a nationwide treatment register that captures operational clinical data for all patients in Denmark who are treated with biologics for severe asthma. Its purpose is to provide an electronic platform to capture operational data and provide a clinical overview of patients with severe asthma, in addition to monitoring the effect of biological treatments and providing research possibilities. The data include clinical information and patient-reported outcome measures[Citation135]. The Swedish National Airway Register (SNAR) was initiated in 2013 and includes data from patients with asthma and chronic obstructive pulmonary disease from primary, secondary and tertiary care; data on patient characteristics, diagnostic interventions, symptom scores, comorbidities and prescribed treatments are reported[Citation136]Citation137 In Finland, the Seinäjoki Adult Asthma Study (SAAS) is a single-centre, 12-year follow-up study of a cohort of patients with new, adult-onset asthma, which aims to increase understanding of the diagnostic process, organisation of long-term asthma care, therapeutic outcomes, prognosis and prognostic factors in this population[Citation138]Citation139. Norway does not yet have a severe asthma registry, but will be commencing a single-centre registry with Haukeland University Hospital starting to enrol patients in late 2021. Alongside NORDSTAR, these asthma registries will provide valuable disease-specific information on an individual patient level that will ultimately be combined with NORDSTAR data to form a dataset with more information on clinical characteristics including biomarkers (such as eosinophils), providing a comprehensive picture of asthma management across the Nordic countries.
Epidemiological data from Nordic countries to date
While the NORDSTAR project is in its infancy, there are epidemiological data from Nordic countries that provide valuable information on the impact of eosinophils in both health and disease, as well as the burden of severe asthma, in these populations.
Impact of elevated eosinophils in the general population
In the general population, a number of Nordic studies have demonstrated deleterious effects associated with elevated eosinophil counts. In the Copenhagen Primary Care Differential Count (CopDiff) database, which holds results from all different cell counts requested by general practitioners in Copenhagen between 2001 and 2010, eosinophil counts of ≥500 cells/µL (0.5 x 109/L) occurred in approximately 4% of routine blood samples[Citation140]. Compared with individuals with a blood eosinophil count of 160 cells/µL (0.16 x 109/L), those with blood eosinophil counts of 300 cells/µL (0.3 x 109/L) had a significantly increased risk of haematological cancer (odds ratio [OR] 4.64; 95% confidence interval [CI] 4.05, 5.33; p < 0.001) and all-cause mortality (OR 1.99; 95% CI 1.88, 2.10; p < 0.001)[Citation140] A second study using data from the same database showed that compared with individuals with a blood eosinophil count of 160 cells/µL (0.16 x 109/L), those with a blood eosinophil count of 300 cells/µL (0.3 x 109/L) had a significantly increased risk of cardiac disease (OR 1.15; 95% CI 1.08, 1.23; p < 0.001), neurological disease (OR 1.11; 95% CI 1.02, 1.20; p = 0.019) and gastrointestinal disease (OR 1.16; 95% CI 1.09, 1.23; p < 0.001)[Citation141]. Moreover, patients with a blood eosinophil count of 750 cells/µL (0.75 × 109/L) had an approximately two-fold risk of respiratory end-organ damage (OR 2.11; 95% CI 1.96, 2.27; p < 0.001) or skin disease (OR 1.88; 95% CI 1.64, 2.15; p < 0.001) compared with those with a blood eosinophil count of 160 cells/µL (0.16 x 109/L).
Impact of elevated eosinophils in the asthma population
Several Nordic studies have also shown the detrimental impact of elevated eosinophil counts in the asthma population. Among patients with asthma from the ongoing, prospective, Copenhagen General Population Study (CGPS), the median blood eosinophil count was 220 cells/µL (0.22 x 109/L) compared with a median count of 160 cells/µL (0.16 x 109/L) among individuals without asthma in the CGPS, indicative of the increased bone marrow drive for eosinophil generation in asthma[Citation38]. Individuals with blood eosinophil counts above 290 cells/µL (0.29 x 109/L) had an elevated risk of both moderate (incidence rate ratio [IRR] 1.28 [95% CI 1.06, 1.55]) and severe asthma exacerbations (IRR 1.55 [1.20, 2.00]) compared with individuals in the CGPS who had blood eosinophil counts <180 cells/µL (0.18 x 109/L)[Citation38]. In the Swedish PAHCER study, patients with asthma and frequent exacerbations had higher blood eosinophil counts than those without exacerbations (460 vs 290 cells/µL)[Citation62]. Moreover, in a Swedish national survey study of children and young adults with asthma, blood eosinophil levels were independently associated with a higher risk of asthma exacerbations[Citation142]. In a Finnish retrospective registry study, high blood eosinophil counts (≥300 cells/µL) were associated with more outpatient visits and lower mortality rates than lower counts (<300 cells/µL), although both groups had similar Charlson Comorbidity Index scores[Citation63].
The burden of severe asthma
A Swedish cohort study conducted in 2017 found that societal costs due to severe asthma were substantial, with mean annual asthma-related costs per patient amounting to €6500, driven mainly by hospitalisations and medications[Citation143[. Notably, comorbid conditions were common in this population, and contributed substantially to per-patient costs. Patients receiving regular treatment with OCS had greater costs and poorer HRQoL than those without regular OCS treatment. The study therefore highlighted a need for improved management and treatment regimens for patients with severe asthma. In a 12-year, prospective, single-centre study conducted in a hospital setting in Finland, 5.9% of patients with adult-onset asthma fulfilled criteria for severe asthma and 2% qualified for anti-IL-5 treatment in that they were using daily medium-to-high dose ICS and LABA, had ≥2 exacerbations in the previous year and a blood eosinophil count of ≥300 cells/µL (0.3 x 109/L) or fraction of exhaled nitric oxide ≥50 ppb [Citation144]. Anti-IL-5 eligibility was associated with frequent healthcare visits, OCS use and work absence due to sickness during the 12-year follow-up period, highlighting the burden of disease in these patients[Citation144].
Future directions
While data from NORDSTAR are still awaited, the project promises to provide a comprehensive picture of the asthma trajectory for patients in Nordic countries that is generalisable to other western countries. It is thought that this will include detailed data on severe eosinophilic asthma and the impact of different biologic treatment approaches in these difficult-to-treat patients. Lessons learned through NORDSTAR and other large asthma registries may be useful in setting up similar programmes in other eosinophilic diseases, although it is noted that disease prevalence for some is much lower.
Conclusion
Much progress has been made in recent years in terms of our understanding of eosinophil biology and eosinophilic diseases; however, knowledge gaps remain. Large-scale, real-world registry data may provide important information regarding the pathophysiology of eosinophilic diseases and clinical characteristics or biomarkers that may allow targeted treatment to ensure optimal outcomes for patients. The structure of the Nordic asthma registry system is unique in the uniformity of data capture across individual registries and countries, providing an opportunity to gather large-scale, patient-level, longitudinal data. This level of clinical information will be helpful for determining how best to target treatment among patients with severe eosinophilic asthma. It is possible that adopting a similar registry structure for other eosinophilic airway diseases may help to advance our understanding of their pathogenesis, progression and effective treatment options in the future.
Author contributions
All authors contributed to the conception and design of this review article, in addition to writing, editing and providing final approval of the submitted version of the article.
Acknowledgments
Editorial support (in the form of writing assistance, including preparation of the draft manuscript under the direction and guidance of the authors, collating and incorporating authors’ comments for each draft, assembling tables and figures, grammatical editing and referencing) was provided by Bianca Paris, PhD, at Fishawack Indicia Ltd, UK, part of Fishawack Health, and was funded by GlaxoSmithKline (GSK).
Disclosure statement
CJ reports personal fees from AstraZeneca, Chiesi Pharma AB, Boehringer Ingelheim, GSK, Novartis, Orion, Sanofi Genzyme and Teva; LB reports lecture fees from AstraZeneca, Boehringer Ingelheim, Chiesi Pharma AB, GSK, Novartis, Sanofi Genzyme and Teva; LL reports personal fees from AstraZeneca, Boehringer Ingelheim, Chiesi, GSK, Mundipharma, Novartis, Orion, Sanofi Genzyme and Teva; HK reports personal fees from AstraZeneca, Chiesi Pharma AB, Boehringer Ingelheim, Novartis, Mundipharma, Sanofi Genzyme, GSK and MSD, and non-financial support from AstraZeneca and Orion Pharma; JK has received lecture fees from AstraZeneca, Boehringer Ingelheim, Chiesi, GSK, Novartis and Orion Pharma, and has participated in advisory boards for AstraZeneca, GSK and Novartis; AA has received lecture fees from AstraZeneca, Berlin Chemie Menarini, Boehringer Ingelheim, Chiesi, GSK, MSD, Norameda, Sanofi and Zentiva, support for meeting attendance from AstraZeneca, Boehringer Ingelheim, Novartis, GSK, Sanofi and Teva, and has participated in advisory boards for AstraZeneca, Boehringer Ingelheim, GSK, Novartis, Sanofi and Teva; VY reports lecture fees from GSK and Sanofi and has participated in advisory boards for AstraZeneca, Boehringer Ingelheim, Chiesi and GSK; BA reports speaker honorarium and/or honorarium for advisory board participation from GSK, AstraZeneca, Chiesi, Boehringer Ingelheim, Novartis and Sanofi-Aventis; MR reports lecture fees from GSK; JH reports lecture fees from AstraZeneca, GSK, MEDA, Schering Plough and MSD, and advisory board participation for GSK, Schering Plough and MEDA; ML and PHH are employees of GSK and own stocks/shares; CP reports personal fees and research grants from AstraZeneca, Chiesi Pharma AB, GSK, Novartis, Sanofi Genzyme and Teva.
Additional information
Notes on contributors
Christer Janson
Christer Janson is a Professor of Respiratory Medicine at Uppsala University, Sweden. His research focuses on improving the prognosis of asthma and COPD through clinical and epidemiological research.
Leif Bjermer
Leif Bjermer is a Senior Professor at the Dept of Respiratory Medicine and Allergology at Skane University Hospital, Lund University, Lund, Sweden.
Lauri Lehtimäki
Lauri Lehtimäki is a Professor of Respiratory Medicine at Tampere University, Finland. His recent research focuses on airway inflammation in asthma, COPD and occupational lung diseases.
Hannu Kankaanranta
Hannu Kankaanranta is Asthma and Allergy Research Professor at the Krefting Research Centre, Gothenburg University, Sweden and Professor of Respiratory Medicine at Tampere University, Finland. His research focuses on adult-onset asthma and severe asthma and the cellular and molecular processes underlying asthma pathophysiology.
Jussi Karjalainen
Jussi Karjalainen is Head of the Allergy Centre at Tampere University, Finland. His research focuses on phenotypes and clinical and occupational outcomes among patients with asthma.
Alan Altraja
Alan Altraja is a Professor of Pulmonary Medicine and Head of the Department of Pulmonology, University of Tartu, Estonia. His research interests include respiratory metabolomics, the mechanisms and phenotypes of obstructive pulmonary diseases, and the diagnosis and management of interstitial and pulmonary vascular diseases and lower respiratory tract infections.
Valentyna Yasinska
Valentyna Yasinska is a physician and specialist in pulmonary medicine and internal medicine at the Department of Respiratory Medicine and Allergy, Karolinska University Hospital, Sweden. Her research interests include non-invasive biomarkers and clinical phenotyping of respiratory diseases including asthma, particularly severe asthma.
Bernt Aarli
Bernt Aarli is Associate Professor at the Department of Clinical Science, University of Bergen, Norway. His research interests include treatment and clinical outcomes of patients with chronic obstructive pulmonary disease.
Madeleine Rådinger
Madeleine Rådinger is a Professor in Immunology at the University of Gothenburg and Chairman of the Krefting Research Centre, Gothenburg, Sweden. Her research focuses on the role of the immune system in subgroups of asthma, including the contribution of immune regulatory microRNAs.
Johan Hellgren
Johan Hellgren is a Professor of Ear, Nose and Throat Diseases at the University of Gothenburg, Sweden. His research focuses on the relationship between upper and lower respiratory tract inflammation based on risk factors and treatment.
Magnus Lofdahl
Magnus Löfdahl is a Study Physician in the Department of Late Development Respiratory and Immunology, AstraZeneca Gothenburg, Sweden. His research focuses on pharmacology of respiratory diseases such as asthma and chronic obstructive pulmonary disease.
Peter H Howarth
Peter Howarth is a Professor Emeritus of Allergy and Respiratory Medicine at the University of Southampton and Global Clinical Scientific Lead (biologics) at GlaxoSmithKline. His research publication record (H index 102) relates to the pathophysiology and pharmacology of airway diseases.
Celeste Porsbjerg
Celeste Porsbjerg is a Clinical Specialist and Professor of Respiratory Medicine at Bispebjerg Hospital and Copenhagen University, Denmark. Her research focuses on the diagnosis and phenotyping of asthma, with a focus on airway pathophysiology and inflammation.
References
- Leru PM. Eosinophilic disorders: evaluation of current classification and diagnostic criteria, proposal of a practical diagnostic algorithm. Clin Transl Allergy. 2019;9(1):1–16.
- Maret-Ouda J, Tao W, Wahlin K, et al. Nordic registry-based cohort studies: possibilities and pitfalls when combining Nordic registry data. Scand J Public Health. 2017;45(17_suppl):14–19.
- Long H, Liao W, Wang L, et al. A player and coordinator: the versatile roles of eosinophils in the immune system. Transfusion Med Hemotherapy. 2016;43(2):96–108.
- Rosenberg HF, Dyer KD, Foster PS. Eosinophils: changing perspectives in health and disease. Nat Rev Immunol. 2013;13(1):9–22.
- Samitas K, Radinger M, Bossios A. Current update on eosinophilic lung diseases and anti-IL-5 treatment. Recent Pat Antiinfect Drug Discov. 2011;6(3):189–205.
- Sehmi R, Smith SG, Kjarsgaard M, et al. Role of local eosinophilopoietic processes in the development of airway eosinophilia in prednisone-dependent severe asthma. Clin Exp Allergy. 2016;46(6):793–802.
- Mjosberg J, Spits H. Human innate lymphoid cells. J Allergy Clin Immunol. 2016;138(5):1265–1276.
- Boberg E, Johansson K, Malmhall C, et al. Interplay between the il-33/st2 axis and bone marrow Ilc2s in protease allergen-induced il-5-dependent eosinophilia. Front Immunol. 2020;11:1058.
- Johansson K, Malmhall C, Ramos-Ramirez P, et al. Bone marrow type 2 innate lymphoid cells: a local source of interleukin-5 in interleukin-33-driven eosinophilia. Immunology. 2018;153(2):268–278.
- Pelaia C, Paoletti G, Puggioni F, et al. Interleukin-5 in the pathophysiology of severe asthma. Front Physiol. 2019;10:1514.
- Kanda A, Yun Y, Bui DV, et al. The multiple functions and subpopulations of eosinophils in tissues under steady-state and pathological conditions. Allergol Int. 2021;70(1):9–18.
- Coden ME, Berdnikovs S. Eosinophils in wound healing and epithelial remodeling: is coagulation a missing link? J Leukoc Biol. 2020;108(1):93–103.
- Mesnil C, Raulier S, Paulissen G, et al. Lung-resident eosinophils represent a distinct regulatory eosinophil subset. J Clin Invest. 2016;126(9):3279–3295.
- Abdala Valencia H, Loffredo LF, Misharin AV, et al. Phenotypic plasticity and targeting of Siglec-F(high) CD11c(low) eosinophils to the airway in a murine model of asthma. Allergy. 2016;71(2):267–271.
- Sastre B, Rodrigo-Munoz JM, Garcia-Sanchez DA, et al. Eosinophils: old players in a new game. J Investig Allergol Clin Immunol. 2018;28(5):289–304.
- Jacobsen EA, Jackson DJ, Heffler E, et al. Eosinophil knockout humans: uncovering the role of eosinophils through eosinophil-directed biological therapies. Annu Rev Immunol. 2021;39:719–757.
- Lee JJ, Jacobsen EA, McGarry MP, et al. Eosinophils in health and disease: the LIAR hypothesis. Clin Exp Allergy. 2010;40(4):563–575.
- Kalinauskaite-Zukauske V, Januskevicius A, Janulaityte I, et al. Expression of eosinophil beta chain-signaling cytokines receptors, outer-membrane integrins, and type 2 inflammation biomarkers in severe non-allergic eosinophilic asthma. BMC Pulm Med. 2019;19(1):158.
- Shult PA, Lega M, Jadidi S, et al. The presence of hypodense eosinophils and diminished chemiluminescence response in asthma. J Allergy Clin Immunol. 1988;81(2):429–437.
- Miyasato M, Tsuda S, Nakama T, et al. Serum levels of eosinophil cationic protein reflect the state of in vitro degranulation of blood hypodense eosinophils in atopic dermatitis. J Dermatol. 1996;23(6):382–388.
- Conesa A, Tassinari P, Rivera H, et al. Hypodense eosinophils: characterization of surface molecule expression. Paper presented at: Allergy and asthma proceedings 2002.
- Marichal T, Mesnil C, Bureau F. Homeostatic eosinophils: characteristics and functions. Front Med (Lausanne). 2017;4:101.
- Yousefi S, Gold JA, Andina N, et al. Catapult-like release of mitochondrial DNA by eosinophils contributes to antibacterial defense. Nat Med. 2008;14(9):949–953.
- Grozdanovic MM, Doyle CB, Liu L, et al. Charcot-Leyden crystal protein/galectin-10 interacts with cationic ribonucleases and is required for eosinophil granulogenesis. J Allergy Clin Immunol. 2020;146(2):377–389 e310.
- Drake MG, Bivins-Smith ER, Proskocil BJ, et al. Human and mouse eosinophils have antiviral activity against parainfluenza virus. Am J Respir Cell Mol Biol. 2016;55(3):387–394.
- Rosenberg HF. Recombinant human eosinophil cationic protein: RIBONUCLEASE ACTIVITY IS NOT ESSENTIAL FOR CYTOTOXICITY(*). J Biol Chem. 1995;270(14):7876–7881.
- Domachowske JB, Dyer KD, Bonville CA, et al. Recombinant human eosinophil-derived neurotoxin/RNase 2 functions as an effective antiviral agent against respiratory syncytial virus. J Infect Dis. 1998;177(6):1458–1464.
- Wen T, and Rothenberg ME. The Regulatory Function of Eosinophils. Microbiol Spectr. 2016;4(5). DOI:10.1128/microbiolspec.MCHD-0020-2015
- Mawhorter SD, Kazura JW, Boom WH. Human eosinophils as antigen-presenting cells: relative efficiency for superantigen- and antigen-induced CD4+ T-cell proliferation. Immunology. 1994;81(4):584–591.
- Varricchi G, Galdiero MR, Loffredo S, et al. Eosinophils: the unsung heroes in cancer? Oncoimmunology. 2018;7(2):e1393134.
- Loktionov A. Eosinophils in the gastrointestinal tract and their role in the pathogenesis of major colorectal disorders. World J Gastroenterol. 2019;25(27):3503–3526.
- Brigger D, Riether C, van Brummelen R, et al. Eosinophils regulate adipose tissue inflammation and sustain physical and immunological fitness in old age. Nat Metab. 2020;2(8):688–702.
- Esser N, Legrand-Poels S, Piette J, et al. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res Clin Pract. 2014;105(2):141–150.
- Wu D, Molofsky AB, Liang HE, et al. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. 2011;332(6026):243–247.
- Liu CL, Liu X, Zhang Y, et al. Eosinophils protect mice from angiotensin-II perfusion-induced abdominal aortic aneurysm. Circ Res. 2021;128(2):188–202.
- Papi A, Brightling C, Pedersen SE, et al. Asthma. Lancet. 2018;391(10122):783–800.
- Jakiela B, Szczeklik W, Plutecka H, et al. Increased production of IL-5 and dominant Th2-type response in airways of Churg-Strauss syndrome patients. Rheumatology (Oxford). 2012;51(10):1887–1893.
- Vedel-Krogh S, Fallgaard Nielsen S, Lange P, et al. Association of blood eosinophil and blood neutrophil counts with asthma exacerbations in the copenhagen general population study. Clin Chem. 2017;63(4):823–832.
- Hartl S, Breyer MK, Burghuber OC, et al. Blood eosinophil count in the general population: typical values and potential confounders. Eur Respir J. 2020;55(5):1901874.
- Fokkens WJ, Lund VJ, Hopkins C, et al. European position paper on rhinosinusitis and nasal polyps 2020. Rhinology. 2020;58(Suppl S29):1–464.
- Kankaanranta H, Lindsay MA, Giembycz MA, et al. Delayed eosinophil apoptosis in asthma. J Allergy Clin Immunol. 2000;106(1 Pt 1):77–83.
- Zhang X, Moilanen E, Kankaanranta H. Enhancement of human eosinophil apoptosis by fluticasone propionate, budesonide, and beclomethasone. Eur J Pharmacol. 2000;406(3):325–332.
- Zhang X, Moilanen E, Adcock IM, et al. Divergent effect of mometasone on human eosinophil and neutrophil apoptosis. Life Sci. 2002;71(13):1523–1534.
- Coden ME, Walker MT, Jeong BM, et al. Beyond Il-5: metabolic reprogramming and stromal support are prerequisite for generation and survival of long-lived eosinophil. Cells. 2021;10(4):815.
- Kankaanranta H, Ilmarinen P, Zhang X, et al. Tumour necrosis factor-alpha regulates human eosinophil apoptosis via ligation of TNF-receptor 1 and balance between NF-kappaB and AP-1. PLoS One. 2014;9(2):e90298.
- Ilmarinen P, Moilanen E, Erjefalt JS, et al. The polyamine spermine promotes survival and activation of human eosinophils. J Allergy Clin Immunol. 2015;136(2):482–484 e411.
- Food and Drug Administration. Fasenra (benralizumab) highlights of prescribing information. 2021; https://den8dhaj6zs0e.cloudfront.net/50fd68b9-106b-4550-b5d0-12b045f8b184/3647bed4-ce91-4fe7-9bc5-32dbee73f80a/3647bed4-ce91-4fe7-9bc5-32dbee73f80a_viewable_rendition__v.pdf.
- Food and Drug Administration. Dupixent (dupilumab) highlights of prescribing information. 2019; https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/761055s012lbl.pdf.
- Food and Drug Administration. Nucala (mepolizumab) highlights of prescribing information. 2021; https://gskpro.com/content/dam/global/hcpportal/en_US/Prescribing_Information/Nucala/pdf/NUCALA-PI-PIL-IFU-COMBINED.PDF.
- Food and Drug Administration. Xolair (omalizumab) highlights of prescribing information. 2020; https://www.gene.com/download/pdf/xolair_prescribing.pdf.
- Food and Drug Administration. Cinqair (reslizumab) highlights of prescribing information. 2019; https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/0761033s010lbl.pdf.
- Menzies-Gow A, Corren J, Bourdin A, et al. Tezepelumab in adults and adolescents with severe, uncontrolled asthma. N Engl J Med. 2021;384(19):1800–1809.
- Holgate S, Casale T, Wenzel S, et al. Reisner C The anti-inflammatory effects of omalizumab confirm the central role of IgE in allergic inflammation. J Allergy Clin Immunol. 2005;115(3):459–465.
- Diver S, Khalfaoui L, Emson C, et al. Effect of tezepelumab on airway inflammatory cells, remodelling, and hyperresponsiveness in patients with moderate-to-severe uncontrolled asthma (CASCADE): a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Respir Med. 2021;9(11):1299–1312.
- Frossing L, Silberbrandt A, Von Bulow A, et al. Airway gene expression identifies subtypes of type 2 inflammation in severe asthma. Clin Exp Allergy. 2021;52:59–69.
- Organization WH Global health risks: mortality and burden of disease attributable to selected major risks. 2009; https://www.who.int/healthinfo/global_burden_disease/GlobalHealthRisks_report_full.pdf. Accessed May, 2021.
- Backman H, Bhatta L, Hedman L, et al. Asthma is still a risk factor for mortality in Sweden and Norway – the nordic epilung study. Eur Respir J. 2019;54:A2778.
- Lemmetyinen R, Karjalainen J, But A, et al. Higher mortality of adults with asthma: a 15‐year follow‐up of a population‐based cohort. Allergy. 2018;73(7):1479–1488.
- Porsbjerg C, Ulrik C, Skjold T, et al. Nordic consensus statement on the systematic assessment and management of possible severe asthma in adults. Eur Clin Respir J. 2018;5(1):1440868.
- Hekking PP, Amelink M, Wener RR, et al. Comorbidities in difficult-to-control asthma. J Allergy Clin Immunol Pract. 2018;6(1):108–113.
- Toppila-Salmi S, Lemmetyinen R, Chanoine S, et al. Risk factors for severe adult-onset asthma: a multi-factor approach. BMC Pulm Med. 2021;21(1):214.
- Erjefalt JS. Unravelling the complexity of tissue inflammation in uncontrolled and severe asthma. Curr Opin Pulm Med. 2019;25(1):79–86.
- Carr TF, Zeki AA, Kraft M. Eosinophilic and noneosinophilic asthma. Am J Respir Crit Care Med. 2018;197(1):22–37.
- Price DB, Rigazio A, Campbell JD, et al. Blood eosinophil count and prospective annual asthma disease burden: a UK cohort study. Lancet Respir Med. 2015;3(11):849–858.
- Janson C, Lisspers K, Stallberg B, et al. Prevalence, characteristics and management of frequently exacerbating asthma patients: an observational study in Sweden (PACEHR). Eur Respir J. 2018;52(2):1701927.
- Viinanen A, Lassenius MI, Toppila I, et al. The burden of adult asthma in Finland: impact of disease severity and eosinophil count on health care resource utilization. J Asthma. 2020;57(10):1092–1102.
- Pavord ID, Korn S, Howarth P, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. 2012;380(9842):651–659.
- Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371(13):1198–1207.
- Ortega HG, Yancey SW, Mayer B, et al. Severe eosinophilic asthma treated with mepolizumab stratified by baseline eosinophil thresholds: a secondary analysis of the DREAM and MENSA studies. Lancet Respir Med. 2016;4(7):549–556.
- Schleich F, Graff S, Nekoee H, et al. Real-word experience with mepolizumab: does it deliver what it has promised? Clin Exp Allergy. 2020 Jun;50(6):687–695.
- McDowell PJ, Diver S, Yang F, et al. Medical research council: refractory asthma stratification programme (RASP-UK consortium). The inflammatory profile of exacerbations in patients with severe refractory eosinophilic asthma receiving mepolizumab (the MEX study): a prospective observational study. Lancet Respir Med. 2021 Oct;9(10):1174–1184.
- Chupp GL, Bradford ES, Albers FC, et al. Efficacy of mepolizumab add-on therapy on health-related quality of life and markers of asthma control in severe eosinophilic asthma (MUSCA): a randomised, double-blind, placebo-controlled, parallel-group, multicentre, phase 3b trial. Lancet Respir Med. 2017;5(5):390–400.
- Bel EH, Wenzel SE, Thompson PJ, et al. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014;371(13):1189–1197.
- Bjermer L, Lemiere C, Maspero J, et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophil levels: a randomized phase 3 study. Chest. 2016;150(4):789–798.
- Bleecker ER, FitzGerald JM, Chanez P, et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting beta2-agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trial. Lancet. 2016;388(10056):2115–2127.
- Castro M, Zangrilli J, Wechsler ME, et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respir Med. 2015;3(5):355–366.
- Corren J, Weinstein S, Janka L, et al. Phase 3 study of reslizumab in patients with poorly controlled asthma: effects across a broad range of eosinophil counts. Chest. 2016;150(4):799–810.
- FitzGerald JM, Bleecker ER, Nair P, et al. Benralizumab, an anti-interleukin-5 receptor α monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2016;388(10056):2128–2141.
- Nair P, Wenzel S, Rabe KF, et al. Oral glucocorticoid-sparing effect of benralizumab in severe asthma. N Engl J Med. 2017;376(25):2448–2458.
- Nair P, Bardin P, Humbert M, et al. Efficacy of intravenous reslizumab in oral corticosteroid-dependent asthma. J Allergy Clin Immunol Pract. 2020;8(2):555–564.
- Goldman M, Hirsch I, Zangrilli JG, et al. The association between blood eosinophil count and benralizumab efficacy for patients with severe, uncontrolled asthma: subanalyses of the Phase III SIROCCO and CALIMA studies. Curr Med Res Opin. 2017;33(9):1605–1613.
- Castro M, Corren J, Pavord ID, et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N Engl J Med. 2018;378(26):2486–2496.
- Humbert M, Beasley R, Ayres J, et al. Benefits of omalizumab as add‐on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): INNOVATE. Allergy. 2005;60(3):309–316.
- Kostikas K, Brindicci C, Patalano F. Blood Eosinophils as biomarkers to drive treatment choices in asthma and COPD. Curr Drug Targets. 2018;19(16):1882–1896.
- Kolmert J, Gomez C, Balgoma D, et al. Urinary leukotriene E4 and prostaglandin D2 metabolites increase in adult and childhood severe asthma characterized by type 2 inflammation. A Clinical Observational Study. Am J Respir Crit Care Med. 2021;203(1):37–53.
- Valentyna Yasinska JK, Zurita J, Quaranta A, et al. Mepolizumab decreases urinary excretion of LTE4 insevere asthma. Eur Respir J. 2020;56(Suppl. 64):2046.
- Bood JR, Sundblad BM, Delin I, et al. Urinary excretion of lipid mediators in response to repeated eucapnic voluntary hyperpnea in asthmatic subjects. J Appl Physiol (1985). 2015;119(3):272–279.
- Alobid I, Bernal-Sprekelsen M, Mullol J. Chronic rhinosinusitis and nasal polyps: the role of generic and specific questionnaires on assessing its impact on patient’s quality of life. Allergy. 2008;63(10):1267–1279.
- Alobid I, Cardelus S, Benitez P, et al. Persistent asthma has an accumulative impact on the loss of smell in patients with nasal polyposis. Rhinology. 2011;49(5):519–524.
- Kohli P, Naik AN, Harruff EE, et al. The prevalence of olfactory dysfunction in chronic rhinosinusitis. Laryngoscope. 2017;127(2):309–320.
- Delemarre T, Holtappels G, De Ruyck N, et al. Type 2 inflammation in chronic rhinosinusitis without nasal polyps: another relevant endotype. J Allergy Clin Immunol. 2020;146(2):337–343. e336.
- McHugh T, Snidvongs K, Xie M, et al. High tissue eosinophilia as a marker to predict recurrence for eosinophilic chronic rhinosinusitis: a systematic review and meta‐analysis. Int Forum Allergy Rhinol. 2018 Dec;8(12):1421–1429.
- Vlaminck S, Vauterin T, Hellings PW, et al. The importance of local eosinophilia in the surgical outcome of chronic rhinosinusitis: a 3-year prospective observational study. Am J Rhinol Allergy. 2014;28(3):260–264.
- Batra PS, Tong L, Citardi MJ. Analysis of comorbidities and objective parameters in refractory chronic rhinosinusitis. Laryngoscope. 2013;123(Suppl 7):S1–11.
- Canonica GW, Malvezzi L, Blasi F, et al. Chronic rhinosinusitis with nasal polyps impact in severe asthma patients: evidences from the Severe Asthma Network Italy (SANI) registry. Respir Med. 2020;166:105947.
- Gevaert P, Omachi TA, Corren J, et al. Efficacy and safety of omalizumab in nasal polyposis: 2 randomized phase 3 trials. J Allergy Clin Immunol. 2020;146(3):595–605.
- Han JK, Bachert C, Fokkens W, et al. Mepolizumab for chronic rhinosinusitis with nasal polyps (SYNAPSE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med. 2021;9:1141–1153.
- Bachert C, Han JK, Desrosiers M, et al. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): results from two multicentre, randomised, double-blind, placebo-controlled, parallel-group phase 3 trials. Lancet. 2019;394(10209):1638–1650.
- Tversky J, Lane AP, Azar A. Benralizumab effect on severe chronic rhinosinusitis with nasal polyps (CRSwNP): a randomized double‐blind placebo‐controlled trial. Clin Exp Allergy. 2021;51:836–844.
- Marchand E, Reynaud-Gaubert M, Lauque D, et al. Idiopathic chronic eosinophilic pneumonia. A clinical and follow-up study of 62 cases. The groupe d’Etudes et de Recherche sur les Maladies” Orphelines” Pulmonaires (GERM” O” P). Medicine (Baltimore). 1998;77(5):299–312.
- Akuthota P, Weller PF. Eosinophilic pneumonias. Clin Microbiol Rev. 2012;25(4):649–660.
- Nakahara Y, Hayashi S, Fukuno Y, et al. Increased interleukin-5 levels in bronchoalveolar lavage fluid is a major factor for eosinophil accumulation in acute eosinophilic pneumonia. Respiration. 2001;68(4):389–395.
- Nakagome K, Nagata M. Possible mechanisms of eosinophil accumulation in eosinophilic pneumonia. Biomolecules. 2020;10(4):638.
- Katoh S, Matsumoto N, Matsumoto K, et al. Elevated interleukin‐18 levels in bronchoalveolar lavage fluid of patients with eosinophilic pneumonia. Allergy. 2004;59(8):850–856.
- Crowe M, Robinson D, Sagar M, et al. Chronic eosinophilic pneumonia: clinical perspectives. Ther Clin Risk Manag. 2019;15:397.
- Marchand E, Cordier J-F Idiopathic chronic eosinophilic pneumonia. Paper presented at: Seminars in respiratory and critical care medicine 2006.
- Brenard E, Pilette C, Dahlqvist C, et al. Real-Life study of mepolizumab in idiopathic chronic eosinophilic pneumonia. Lung. 2020;198(2):355–360.
- Cottin V, Bel E, Bottero P, et al. Respiratory manifestations of eosinophilic granulomatosis with polyangiitis (Churg-Strauss). Eur Respir J. 2016;48(5):1429–1441.
- Furuta S, Iwamoto T, Nakajima H. Update on eosinophilic granulomatosis with polyangiitis. Allergol Int. 2019;68(4):430–436.
- Mahr A, Moosig F, Neumann T, et al. Eosinophilic granulomatosis with polyangiitis (Churg-Strauss): evolutions in classification, etiopathogenesis, assessment and management. Curr Opin Rheumatol. 2014;26(1):16–23.
- Greco A, Rizzo MI, De Virgilio A, et al. Churg-Strauss syndrome. Autoimmun Rev. 2015;14(4):341–348.
- Baldini C, Talarico R, Della Rossa A, et al. Clinical manifestations and treatment of Churg-Strauss syndrome. Rheum Dis Clin North Am. 2010;36(3):527–543.
- Saku A, Furuta S, Hiraguri M, et al. Longterm outcomes of 188 Japanese patients with eosinophilic granulomatosis with polyangiitis. J Rheumatol. 2018;45(8):1159–1166.
- Lyons PA, Peters JE, Alberici F, et al. Genome-wide association study of eosinophilic granulomatosis with polyangiitis reveals genomic loci stratified by ANCA status. Nat Commun. 2019;10(1):5120.
- Khoury P, Grayson PC, Klion AD. Eosinophils in vasculitis: characteristics and roles in pathogenesis. Nat Rev Rheumatol. 2014;10(8):474–483.
- Manka LA, Guntur VP, Denson JL, et al. Efficacy and safety of reslizumab in the treatment of eosinophilic granulomatosis with polyangiitis. Ann Allergy Asthma Immunol. 2021;126(6):696–701 e691.
- Martinez-Rivera C, Garcia-Olive I, Urrutia-Royo B, et al. Rapid effect of benralizumab in exacerbation of severe eosinophilic asthma associated with eosinophilic granulomatosis with polyangiitis. BMC Pulm Med. 2021;21(1):35.
- Coppola A, Flores KR, De Filippis F. Rapid onset of effect of benralizumab on respiratory symptoms in a patient with eosinophilic granulomatosis with polyangiitis. Respir Med Case Rep. 2020;30:101050.
- Nanzer AM, Dhariwal J, Kavanagh J, et al. Steroid-sparing effects of benralizumab in patients with eosinophilic granulomatosis with polyangiitis. ERJ Open Res. 2020;6(4):00451–2020.
- Guntur VP, Manka LA, Denson JL, et al. Benralizumab as a steroid-sparing treatment option in eosinophilic granulomatosis with polyangiitis. J Allergy Clin Immunol Pract. 2021;9(3):1186–1193 e1181.
- Padoan R, Bianchi FC, Marchi MR, et al. Benralizumab as a glucocorticoid-sparing treatment option for severe asthma in eosinophilic granulomatosis with polyangiitis. J Allergy Clin Immunol. 2020;8(9):3225–3227. e3222.
- Takenaka K, Minami T, Yoshihashi Y, et al. Decrease in MPO-ANCA after administration of benralizumab in eosinophilic granulomatosis with polyangiitis. Allergol Int. 2019;68(4):539–540.
- Butt NM, Lambert J, Ali S, et al. Guideline for the investigation and management of eosinophilia. Br J Haematol. 2017;176(4):553–572.
- Valent P, Klion AD, Horny H-P, et al. Contemporary consensus proposal on criteria and classification of eosinophilic disorders and related syndromes. J Allergy Clin Immunol. 2012;130(3):607–612. e609.
- Kahn JE, Groh M, Lefèvre G. (A critical appraisal of) classification of hypereosinophilic disorders. Front Med (Lausanne). 2017;4:216.
- Klion AD, Bochner BS, Gleich GJ, et al. Approaches to the treatment of hypereosinophilic syndromes: a workshop summary report. J Allergy Clin Immunol. 2006;117(6):1292–1302.
- Moller D, Tan J, Gauiran DTV, et al. Causes of hypereosinophilia in 100 consecutive patients. Eur J Haematol. 2020;105(3):292–301.
- European Medicines Agency. Mepolizumab for the treatment of hypereosinophilic syndrome. 2010; https://www.ema.europa.eu/en/documents/orphan-designation/eu/3/04/213-public-summary-positive-opinion-orphan-designation-mepolizumab-treatment-hypereosinophilic_en.pdf. cited Mar 2021.
- Roufosse FE, Goldman M, Cogan E. Hypereosinophilic syndromes. Orphanet J Rare Dis. 2007;2(1):1–12.
- Curtis C, Ogbogu P. Hypereosinophilic syndrome. Clin Rev Allergy Immunol. 2016;50(2):240–251.
- Khoury P, Abiodun AO, Holland-Thomas N, et al. Hypereosinophilic syndrome subtype predicts responsiveness to glucocorticoids. J Allergy Clin Immunol. 2018;6(1):190–195.
- Ogbogu PU, Bochner BS, Butterfield JH, et al. Hypereosinophilic syndrome: a multicenter, retrospective analysis of clinical characteristics and response to therapy. J Allergy Clin Immunol. 2009;124(6):1319–1325. e1313.
- US National Library of Medicine. A phase 3 study to evaluate the efficacy and safety of benralizumab in patients with Hypereosinophilic Syndrome (HES) (NATRON). 2021; https://clinicaltrials.gov/ct2/show/NCT04191304. cited Jul, 2021.
- Roufosse F, Kahn JE, Rothenberg ME, et al. Efficacy and safety of mepolizumab in hypereosinophilic syndrome: a phase III, randomized, placebo-controlled trial. J Allergy Clin Immunol. 2020;146(6):1397–1405.
- Hansen S, Hilberg O, Ulrik CS, et al. The Danish severe asthma register: an electronic platform for severe asthma management and research. Eur Clin Respir J. 2020;8(1):1842117.
- Stridsman C, Konradsen JR, Vanfleteren L, et al. The Swedish National Airway Register (SNAR) development, design and utility to date. Eur Clin Respir J. 2020;7(1):1833412.
- The Nordic Severe Asthma Network. 2021; https://nordstar-nsan.com/. cited 2021 Jun 29.
- Kankaanranta H, Ilmarinen P, Kankaanranta T, et al. Seinajoki Adult Asthma Study (SAAS): a protocol for a 12-year real-life follow-up study of new-onset asthma diagnosed at adult age and treated in primary and specialised care. NPJ Prim Care Respir Med. 2015;25:15042.
- Geale K, Darabi H, Lindh M, et al. NORDSTAR: paving the way for a new era in asthma research. Eur Respir J. 2020;55(4):1902476.
- Andersen CL, Siersma VD, Hasselbalch HC, et al. Association of the blood eosinophil count with hematological malignancies and mortality. Am J Hematol. 2015;90(3):225–229.
- Bjerrum OW, Siersma V, Hasselbalch HC, et al. Association of the blood eosinophil count with end-organ symptoms. Ann Med Surg (Lond). 2019;45:11–18.
- Mogensen I, Alving K, Jacinto T, et al. Simultaneously elevated FeNO and blood eosinophils relate to asthma morbidity in asthmatics from NHANES 2007-12. Clin Exp Allergy. 2018;48(8):935–943.
- Jansson SA, Backman H, Andersson M, et al. Severe asthma is related to high societal costs and decreased health related quality of life. Respir Med. 2020;162:105860.
- Ilmarinen P, Tuomisto LE, Niemela O, et al. Prevalence of patients eligible for anti-il-5 treatment in a cohort of adult-onset asthma. J Allergy Clin Immunol Pract. 2019;7(1):165–174 e164.